| Pages:
1
2 |
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Suitable manometer
What's the best and or simplest way to measure the vacuum level?
Some pumps have vacuum gauges on them, are these suitable?
Trying to conceptualize how a vacuum distillation is pieced together, and right now I'm perplexed by the vacuum
[Edited on 1/12/2023 by Yttrium2]
[Edited on 1/12/2023 by Yttrium2]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|

|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
A gauge that reads from zero to about 30 Torr would be fine AFAIK.
Some vacuum distilations are done at very low pressure if the substance is very temperature sensitive.
There is a simple vacuum controller attached which may be very useful.
Yob
Attachment: 10.1063_1.1137334.pdf (387kB)
This file has been downloaded 146 times
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
A common vacuum gauge.
One of these

I guess you could bend a glass tube to a U-shape using a torch and fill it with liquid mercury.
But you would not know how much vacuum each level represents without calibrating it with another vacuum gauge.
[Edited on 2023-1-23 by Mateo_swe]
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Quote: Originally posted by Mateo_swe  | A common vacuum gauge.
I guess you could bend a glass tube to a U-shape using a torch and fill it with liquid mercury.
But you would not know how much vacuum each level represents without calibrating it with another vacuum gauge.
[Edited on 2023-1-23 by Mateo_swe] |
You just need an accurate way to measure length.
10 inHg = 10 inches of mercury.
Diameter of the tube is irrelevant.
"You can't do that" - challenge accepted
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
I guess what I was meaning to ask in my initial thread to be specific is this:
Is this how a vacuum distillation is done?
Adjust vacuum to level or more than compound of interest...
Does vacuum reach a certain vacuum level and stop pumping or is it continuously pumping to keep air evacuated and the vacuum level maintained?
Sorry, I was very vague here
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
The type of vacuum in the picture is a water aspirator-based device.
You add water and ice to the container, and it starts pumping.
With room-temperature water you will get about 15inHg of vacuum
It runs continuously.
From what I have read, most people continuously run their vacuum when doing distillation with a soft vacuum.
"You can't do that" - challenge accepted
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
"I guess you could bend a glass tube to a U-shape using a torch and fill it with liquid mercury.
But you would not know how much vacuum each level represents without calibrating it with another vacuum gauge."
You would measure the difference in height of the two Hg coloumbs (or am I missing something).
You could use vacuum oil instead of Hg. Not toxic, cheaper, less dense and therefor would give more resolution.
See the belljar.net look in publications, the first five years for an oil manometer.
NOTE: I don't have any shares in the Bell Jar. 
Yob
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
@yobbo II
You are correct; the difference in height of the two columns is the pressure in the system; one arm of the U-tube is open to the atmosphere, and the
other is connected to the system. Of
course the height of the U-tube should be somewhat more than 760 mm to allow for an ambient atmospheric pressure greater than 1 atmosphere.
A quick search yielded this company that has many different manometers:
https://www.globaltestsupply.com/product/dwyer-1221-8-w-m-ma...
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
i use a gauge off of an old refrigeration charging rig which i got a pawn shop for next to nothing, reads down to 30 inches and is fairly accurate.
be VERY careful if you start pulling vacuum on things--warm liquids can suddenly flash boil, and NEVER NEVER pull vacuum on a standard erlenmyer
flask. i KNOW better and yet thought that i could quickly do a vacuum filtration. imploded a 2 liter flask with hot aqua regia in it.
don't be me!
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Would this be useful in the conceptualization of the u tube?
https://phet.colorado.edu/sims/html/under-pressure/latest/un...
It is interesting, coming from the world of a scuba diver learning how much an atmosphere was and how much pressure there was with how much depth, and
then thinking about how that all goes out the window if we are dealing with gasoline on Jupiter.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
What impact does gravity have on the depth pressure relationship?
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
So if a DIY U-shaped glass manometer filled with merqury is made to be used as a vacuum gauge it must be at least 30 inches tall (about 760mm).
Doesn it matter how much mercury is put inside?
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
If the sealed end of a u-tube is full of mercury,
ie no air = no air pressure = vacuum
If you only want to measure low pressure
eg below 20mmHg then you only require a short u-tube.
At the open end of the u-tube, if the pressure (of whatever is attached) is zero,
mercury will be at the same height in both sides of the u-tube = half way up the ruler = 0 mmHg,
With a pressure of 20 mmHg the mercury will rise 10 mm in the sealed side,
and fall 10 mm in the other (open) side
etc.
So you'd only need a common 12 inch glass tube for 0 to 100 mmHg.
Plus the mercury.
I guess vacuum oil might work for (10x) lower pressures
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Any liquid will work as long as you correct the scale
And stay above the vapor pressure
Water is well studied and cheap.
Glycol is used in the gauge showen above
"You can't do that" - challenge accepted
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | If the sealed end of a u-tube is full of mercury,
ie no air = no air pressure = vacuum
If you only want to measure low pressure
eg below 20mmHg then you only require a short u-tube.
At the open end of the u-tube, if the pressure (of whatever is attached) is zero,
mercury will be at the same height in both sides of the u-tube = half way up the ruler = 0 mmHg,
With a pressure of 20 mmHg the mercury will rise 10 mm in the sealed side,
and fall 10 mm in the other (open) side
etc.
So you'd only need a common 12 inch glass tube for 0 to 100 mmHg.
Plus the mercury.
I guess vacuum oil might work for (10x) lower pressures |
These sorts of (mercury) manometers are great, but you need to be careful with them.
A sudden change in pressure will slam the mercury into the end of the tube and that can break the glass.
Having a restriction in the middle of the "U" will help.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
What is the appropriate manometer I can fill with water that would also work with Hg? -- That I can take measurements on -- to better understand the
relationships between variables, and the usage of tools to make those measurements?
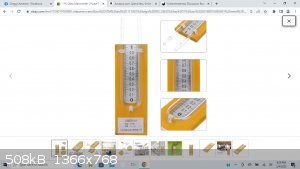
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
(I guess I have to know at what vacuum level or whatever my stuff comes off at, get the right gauged, or column reading level in -- what is the
frequently referred to term? inches of mercury, or was it mm??? Why does the above poster say that the mercury manometer is great? I was previously
thinking that they were necessary, for some reason, I forget what that reason was.
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
The round common vacuum gauges (the pic i posted in my post erlier in this thread) is the easiest to use and are cheap.
I dont think they are super exact but they dont have the risk of mercury spill that a U-shaped glass gauge has if a sudden fast pressure change is
happening like a hose disconnecting.
I dont know how good the are at handling chemicals, the U-shaped mercury tube is probably much better in that regard.
The U-shaped type can also be filled with other liquids but they must be made longer i think and preferably calibrated with another vacuum gauge.
There are also the digital vacuum gauges, these are very good but cost a bit of money especially a good brand one.
These are also sensitive for corrosive chemicals but is very handy to use to calibrate/check any other vacuum gauges you have.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Quote: Originally posted by Mateo_swe  |
There are also the digital vacuum gauges, these are very good but cost a bit of money especially a good brand one.
These are also sensitive for corrosive chemicals but is very handy to use to calibrate/check any other vacuum gauges you have. |
point noted
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
What are these for?
Do they have any clever repurposed applications?
[Edited on 2/5/2023 by Yttrium2]
[Edited on 2/5/2023 by Yttrium2]
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Those sets with vacuum pump and a vacuum vessel is for degassing epoxy and polyurethane glues so there is no bubbles in it when it hardens.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
so for a vacuum distillation setup I need:
Pump, gauge, vacuum trap flask, tubing, hosing clamps?
-- What are some cool physics experiments I could do with the above listed!?
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
Some general points.
Vacuum and pressure are measured in several different units.
The difference between atmospheric pressure and a vacuum can be in mm of mercury (mmHg), inches of mercury ("Hg) or inches of water, and that is just
in the US. 25.4 mmHg equals 1"Hg equals 13.5"water. The difference between the mercury and the water measurement is due to mercury being 13.5 times
denser.
A pressure difference of one atmosphere is 760mmHg, or 30"Hg, or about 32 feet of water, so if you wanted to measure a total vacuum and use water in
your manometer it would have to be more than 32 ft tall. On top of that is the vapor pressure of water, it would start to boil at room temperature at
about 29mmHg.
An aspirator can generate about 26-27 "Hg of vacuum. Mechanical pumps can get lower. Some types of mechanical vacuum pumps should not be used if the
vapor is corrosive or otherwise damaging to the pump.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Thanks for clarification everybody, I've got enough here to digest now for a while.
|
|
|
| Pages:
1
2 |