blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Zinc voltammetry: some problems
I want to develop a method for voltammetric determination of a well known metal. I started off using the Daniell cell as a model:
$$Zn(s) | Zn^{2+}(aq) || Cu^{2+}(aq,sat) | Cu(s)$$
I built a Daniell cell with a CuSO4/Cu reference electrode (about 7 mL electrolyte), 50 mL anodic compartment and used a plate of 99% Zn as
anode. Stock solutions (but not standardised) of ZnSO4 were prepared as 'analytes'.
Problems with reproducibility and time to equilibrium forced me to scale everything down, with a RE of about 1.5 mL and an anodic compartment volume
of about 5 mL: [see next post]
I also tried stirring and flushing with Ar welding gas but neither made any difference.
With this configuration I measured the EMF (at 19 C) for five Zn2+ stock solutions and their replica.
With Nernst we get:
$$\color{red} {\underbrace{E=E° -\dfrac{0.059}{n} \log_{10} \gamma [Zn^{2+}]}_{\text{Applicable at only 298K}}} \label{Nernst Short}$$
The following picture says more than a thousand words:
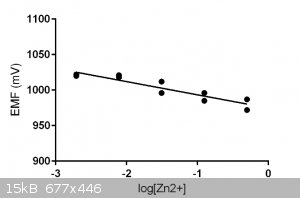
The problems are:
* poor reproducibility
* long equilibrium times: 10 mins or longer.
Both the intercept and the slope are in the right ballpark.
[Edited on 2-2-2023 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Cell and electrodes:
 

[Edited on 2-2-2023 by blogfast25]
|
|
|
RU_KLO
Hazard to Others
  
Posts: 147
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
It is possible that the zinc plate is covered on oxide and should be polished? (from your pictures)
(once I tried to check for zinc on a zinc plate with CuSO4 solution (it contained another reagent - cannot recall which was. The test for zinc,is to
get a black spot when you add a drop of the solution on zinc)
I know for sure that it was Zinc but the test did not work (no black spot)
When I polished the Zinc, the test was succesful (black spot)
so maybe oxide maybe is interfering? (wearing of on each test, so different results)
Note: I do not have knowledge of Voltammetry.
Go SAFE, because stupidity and bad Luck exist.
|
|
|
RU_KLO
Hazard to Others
  
Posts: 147
Registered: 12-10-2022
Location: Argentina
Member Is Offline
|
|
It is possible that the zinc plate is covered on oxide and should be polished? (from your pictures)
(once I tried to check for zinc on a zinc plate with CuSO4 solution (it contained another reagent - cannot recall which was. The test for zinc,is to
get a black spot when you add a drop of the solution on zinc)
I know for sure that it was Zinc but the test did not work (no black spot)
When I polished the Zinc, the test was succesful (black spot)
so maybe oxide maybe is interfering? (wearing of on each test, so different results)
Note: I do not have knowledge of Voltammetry.
Go SAFE, because stupidity and bad Luck exist.
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
What is your separator?
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Are you sure that this is voltammetry? It look like potentiometry to me. If I understand it correctly, you just measured how potential changed with
decreasing Zn2+ concentration. If you want to do voltammetry, you must apply changing pontential and measure change in current.
https://chem.libretexts.org/Bookshelves/Analytical_Chemistry...
Nice experiment though. Do you consider use similar setup for potentiometric titration?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bedlasky  | Are you sure that this is voltammetry? It look like potentiometry to me.
Nice experiment though. Do you consider use similar setup for potentiometric titration? |
Voltammetry/potentiometry... you know?   
No, it's potentiometry, definitely. 
I wanted to use the setup for standard addition and titration methods but with such poor reproducibility that's out of the question.
I'm now waiting for an Ag/AgCl ref. electrode, maybe it will give better results?
[Edited on 11-2-2023 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I clean the Zinc plate by dunking it into 2 M HCl for 5', prior to a run of measurements. Bubbles of H2 then occur. So oxide cannot be the
problem.
[Edited on 11-2-2023 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
An Aga agar gel, 1 M NaCl, at the bottom of the Cu ref. electrode.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The new Ag/AgCl reference electrode has arrived:

So the experiment above was repeated, albeit with a slightly better grade of ZnSO4.
The results were very much similar to the first run:
* reproducibility was slightly better but not brilliant
* equilibrium times remained long: 5 mins and longer
The slope was -8.2+/-2.2 mV when acc. Nernst (see above) it should be about 28 mV. I can't explain the discrepancy. The lower slope
means of course reduced sensitivity.
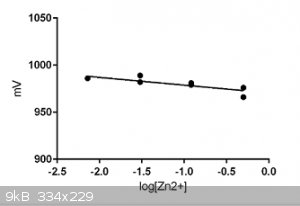

|
|
|