| Pages:
1
..
4
5
6
7
8
9 |
Roger86
Harmless

Posts: 31
Registered: 17-8-2011
Member Is Offline
Mood: No Mood
|
|
Tried to search for that book on pdf form but couldnt find it, do you have a link?
Not that i have given up on beetroots, but i seriously would like to read that, especially since it's been done on 19th century technology, what
should make it simple to do at home
|
|
|
krystaljjang90
Harmless

Posts: 2
Registered: 2-10-2011
Member Is Offline
Mood: No Mood
|
|
hi all..
I'm a chemical engineering student and I need to design malonic acid plant for my plant design subject..
i'll be really grateful if anyone can anyone tell me about the industrial process of malonic acid..
tq...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Hi krystal,
Have you checked out the Kirk-Othmer Encycopedia of Chemical Technology and Ullman's Encyclopedia of Industrial Chemistry? Most
university libraries should have one or both.
[Edited on 3-10-2011 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
krystaljjang90
Harmless

Posts: 2
Registered: 2-10-2011
Member Is Offline
Mood: No Mood
|
|
yeah I've checked them n shortlisted 2 processes which are alkaline saponification of cyanoacetic acid and acidic
saponification of chloroacetic acid..i've spent days searching patents and journals but it was not enough..
can you recommend some links or maybe do you know about these processes and the equipments involved??..tq
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Sorry, other than the patents I wouldn't know where else to look.
I'm just guessing that malonic acid is being produced today by the usual Indian and Chinese bulk chemical producers. Businesses don't usually publish
details on their processes for obvious reasons.
------------------------------------------------------------
Edit: Here's another possibility. Search the archives of these magazines:
Chemical Engineering Progress
Chemical & Engineering News
Chemical Engineering
[Edited on 4-10-2011 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sauron  | BTW red P is not mandatory for chlorination of acetic acid, it merely accelerates the rate of the chlorination. Direct chlorination is UV mediated so
you need reliable sunlight or preferably a UV reactor. OR chlorinate with CuCl2 (see thread) or N-chlorosuccinimide, both of which monochlorinate the
alpha position of carboxylic acids I think. NBS does for sure and bromoacetic acid would work just as well,
|
May I ask, to which thread do you refer (wrt CuCl2)? I've not been able to find it.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Once again I find myself reading a long an painful thread with very little useful content. I think we have established there are only really two
practical methods to malonic acid for the amateur. The chloroacetic acid + alkali cyanide and the oxidation of malic acid. The first of these suffers
from the availability of sodium and potassium cyanide and the second from the fact that the published proceedure are old and often don't appear to
work. Other procedures such as the hydrolysis of malononitrile or the oxidation of 1,3 propandiol suffer from the difficult of obtaining the starting
material again (if you can get these compounds you can probably buy malonic acid anyway).
I can't add much more to the first technique but the second looks like fertile ground for investigation, malic acid is easily available from home brew
suppliers etc and is cheap; there are dozens of easily available oxidizing agents on the high street or the internet.
I am not going to go through my experiments in detail I have attached a copy of my prelimenary experimental findings for anyone who is interested and
I have so far only looked at the oxidation and precipitation as calcium malonite. I intend to try the work up of the precipitates in the near future.
However, a summary of the work so far is never the less interesting. I tried oxiding dl malic acid with the following reagents:
Sodium dichromate
Sodium hypochlorite
Hydrogen peroxide and tungstate catalyst
sodium chlorate and vanadium pentoxide catalyst
From the attached file you will see that sodium dichromate and hydrogen peroxide did not work for certain as no calcium salt precipitated. The
tungstate catalyst was used with hydrogen peroxide because I have somewhere in my documents a reference to Fe salts and H2O2 being used to oxidize
malic acid to oxaloacetic acid, though there papers posted on this thread that describe the oxidation of citric acid to malonic acid via several
possible intermediates so this route is not closed but clearly requires different conditions.
Sodium chlorate with V2O5 gave only a slight precipitate inspite of the apparently fairly vigorous reaction so once again oxaloacetic acid may be the
main product.
It would appear on the basis of the amount of precipitate formed that the sodium hypochlorite method is by far the most successful. However, towards
the end of the addition of the hypochlorite the solution became cloudy and heavy mobile droplet formed in the liquid and the whole smelled strongly of
chloroform. This suggests that towards the end the conditions become alkaline and the haloform reaction becomes an important side reaction. Experiment
with buffering with HCl towards the end were tried to prevent this but there is clearly much scope for improvement. That said the reactants are cheap
and easily obtained so modest yields may not be an issue.
Excessive oxidation could theoretically produce oxalic acid which would also precipitate with calcium salts so the formation of a precipitate with
calcium chloride does not prove that malonic acid was formed, however, the absence of a precipitate indicates that it was not formed.
One further point is that when using calcium chloride to precipitate free malonic acid hydrochloric acid is liberated potentially dropping the pH to a
point where calcium malonate will no longer precipitate. Clearly there is much scope for the optimisation of this method.
The precipitates from the hypochlorite oxidation experiments where subjected to the diphenylamine-sulphuric test for oxalates and gave practically
negative results indicating very little calcium oxalate to be present.
Attachment: Malic acid Oxidation Experiments.docx (18kB)
This file has been downloaded 1264 times
[Edited on 1-11-2012 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have recently returned to the topic of OTC synthesis of Malonic acid both from the oxidation of malic acid and via cyanoacetic acid. While looking
for reasonably recent online papers concerning the former process I came across this one:
Effect of MnII ions on the oxidation of the oxidation of malic and oxaloethanoic acids by aqueous HCrO4-
Zaheer Khan and Kabir-ud-Din, Transition Metal Chemistry, v26, p672-678, 2001
This paper explores the catalytic affect of MnII on this reaction but I could only access the first page, however, on this page there is a claim that
following a measurement of the amount of CO2 evolved the reaction must approximate to:
12HCrO4- + 9C4H6O5 + 48H+ ==> 12CrIII + 2C2H4O2(acetic acid) + 7C3H4O4 +11CO2 +39H2O
(14H2O in the paper but this is clearly a typo)
This is a higher ratio of oxidant than I used above but I would still have expected some malonic acid to have been precipitated. I will investigate
this further when I get chance.
|
|
|
bfesser
|
Thread Pruned
20-2-2014 at 05:13 |
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
I know those 2 methods ... and I also tryed the old clasic method (acetic acid --> cyanoacetic acid --> malonic acid) and I observe that it
consume a lot of cyanide salts, wich are hard to make (by me), as I'm a home-laboratory-chemist.
But something come into my mind ... there might be a way to obtain malonic acid more easy and in a much more pure form (not using chromates,
permanganates, cyanides...)
The process look like this:
CH3-COOH(liquid) + Cl2(gas) --(intense blue light, traces of acetic anhydride and some CCl4, 60-70 *C, probably on an ethanol bath)--> Cl-CH2-COOH
+ HCl
HCOOH(gas) + Cl-CH2-COOH (gas) --(traces of H2O and AlCl3 cristals deposited in a glass tube at 190-200*C)--> HOOC-CH2-COOH + HCl
It is corect ? ... I hope it is, because I will test this theory 
[Edited on 13-4-2014 by DoctorZET]
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
As soon I post that, I can see a big problem : the malonic acid starts to decarboxylate at about 70*C forming acetic acid ... sooo...at about 190*C
should be already decomposed into CO2 and acetic acid. 
So if I want to do the Friedel-Kraft alkylation reaction in the gas phase (because I want to have a fast reaction), I must do it at a lower pressure.
But I also could do it at max.60-70*C for a few hours, with a lot of AlCl3(better use FeCl3) added and excess of formic acid...
Then I can distill the excess of formic acid.
Sounds good...
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
An easier way to make malonic acid?
I read the entire thread and as far as i can tell nobody suggested this but correct me if im wrong.
Simply react allyl alcohol with a hydrogen halide or possibly a halide and aluminium to get allyl halide (1-halo-2-Propene) then after drying it
thoroughly react with magnesium in diethyl ether or THF to get the grignard reagent then of coarse either add dry ice or bubble CO2 through the
grignard reagent to get 3-buteneoic acid after acidification with H2SO4, then obviously reflux with potassium dichromate or permangenate and
neutralize then distil and bobs your uncle your done.
CH2CHCH3-MgBr + CO2 ------> CH2CHCH3COOH
CH2CHCH3COOH + K2Cr2O7 ------> COOHCH2COOH
Allyl alcohol can easily be synthesized OTC from oxalic acid and glycerol, the only annoying part that i can see about this method would be drying the
allyl halide but i mean fractional distillation over a dessicant such as calcium chloride or hell even molecular sieves then maybe left to dry over
some sodium overnight not really that difficult.
Also given that butanoic acid has a boiling point of 164*C i would speculate that buntenoic acid would have a similar boiling point thus making pretty
much all the workups piss easy.
I just dont get why nobody else thought of this.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Allyl alcohol synthesized from oxalic acid and glycerol ???
How the heck can that happen, you would have to be mad to think that was possible were my first thoughts LOL
Well I checked apparently it is correct but the yield is low. I am still looking for the detailed mechanism.
Ok a little off topic and its probably been posted already:
https://erowid.org/archive/rhodium/chemistry/allylalcohol.ht...
"Experimental
Allyl Alcohol from Glycerol and Oxalic Acid
A mixture of 500g anhydrous oxalic acid and 500g of glycerol was heated in a partial vacuum on a water bath for 4-5h (or longer) until formic acid
ceased to distill over. The mixture was then gradually heated to 240°C under ordinary pressure, the flask being fitted with a fractionating column.
At 220-225°C, CO2 was given off and a mixture of approximately equal amounts of allyl alcohol and allyl formate distilled over leaving in the
distillation flask a residue containing somewhat 50% of the glycerol originally used. Practically no acrolein was produced. The distillate was treated
with 50g NaOH in 1000ml water (to hydrolyze the formate), allowed to stand for 12h at room temp, and finally distilled. The first 300 ml of distillate
contained all the allyl alcohol, which after fractionation yielded 200-210g of a allyl alcohol/water mixture (bp 87-88°C) which may be dehydrated
using anhydrous potassium carbonate yielding approximately 150g of anhydrous allyl alcohol."
[Edited on 3-10-2016 by wg48]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
The triethylorthoformate method posted here looks like a better way to go about allyl alcohol preparation, the thread also goes into more detail
regarding the oxalic acid glycerol method although the mechanism still seems obscure. I do rest my case however that allyl alcohol should be fairly
straightforward and OTC to prepare and then the grignard carbonation and oxidation of the double bond to finish up.
http://www.sciencemadness.org/talk/viewthread.php?tid=6274
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  | The triethylorthoformate method posted here looks like a better way to go about allyl alcohol preparation, the thread also goes into more detail
regarding the oxalic acid glycerol method although the mechanism still seems obscure. I do rest my case however that allyl alcohol should be fairly
straightforward and OTC to prepare and then the grignard carbonation and oxidation of the double bond to finish up.
http://www.sciencemadness.org/talk/viewthread.php?tid=6274 |
Well triethyl orthoformate route to allyl alcohol may be cleaner and more productive but it looks a long way from OTC.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
As far as I'm aware, the exact mechanism for the reaction isn't actually known; however, if you're having trouble trying to come up with a way to get
from oxalic acid and glycerol to allyl alcohol (it's a little tricky), I've drawn a plausible mechanism that is actually based on some recent
literature where deuterium labeling was used:
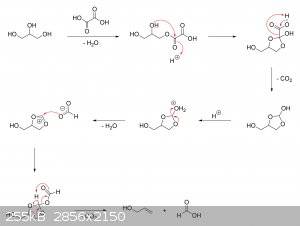
The oxalic acid and glycerol initially react to give a mono-oxalate ester that, upon ring closure, gives
2-hydroxy-4-(hydroxymethyl)-1,3-dioxolan-2-carboxylic acid. This unstable dioxolane ring system then undergoes thermal decarboxylation to the more
stable 4-(hydroxymethyl)-1,3-dioxolan-2-ol, which is in equilibrium with both glycerol mono-formate isomers as well as their hydrolysis products,
glycerol and formic acid:
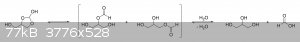
The dioxolane form of glycerol mono-formate then reacts with some of the formic acid produced by the hydrolysis shown above to give a
resonance-stabilized carbocation, which gets attacked by the resulting formate ion to give 4-(hydroxymethyl)-1,3-dioxolan-2-formate. The formate ester
undergoes a final thermal decarboxylation reaction to give both the desired allyl alcohol as well as give back the formic acid that was consumed.
Additionally, it's also possible that, instead of attacking the electrophilic carbon, the formate ion instead abstracts the acidic proton to give an
unstable carbene that immediately decomposes into carbon dioxide and allyl alcohol:
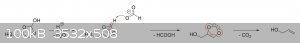
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=13122
There are now a few sources of 1,3-propanediol available as a cosmetic ingredient.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
And also available as antifreeze for solar systems. (At least in the EU.) Not as cheap as ethylene-glycol or 1,2-propane-diol and not so common in
every hardware store, but for a 10 or 20 liters can the price is not bad.
(The reason is reportedly 1,3-propane-diol whitstands better the 150+ Celsius range encountered in solar systems than other diols.)
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Perhaps treating amino acids with TCCA or NaOCl would yield malonic acid.
https://www.sciencemadness.org/whisper/viewthread.php?tid=32...
https://www.sciencemadness.org/whisper/viewthread.php?tid=29...
It looks like the mono-chloroamine of aspartic acid will decompose into an aldehyde and dichloroamine will produce the nitrile.
[Edited on 9-10-2016 by mr.crow]
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I had a somewhat long synthesis in mind from aspirin, ethyl bromide, sodium ethoxide, and hydrogen peroxide:
acetylsalicylic acid + NaOEt + EtOH + ∆ >> sodium acetylsalicylate + EtOH (g) i.e. just make the sodium salt alkoxide is used here or use
something else
sodium acetylsalicylate + EtBr >> NaBr + ethyl acetylsalicylate
ethyl acetylsalicylate + NaOEt (dry) >> 4-hydroxycoumarin (2,4-dioxodihydrobenzopyran) [1]
4-hydroxy-2-chromone + NaOH + H2O + H2O2 >> sodium 2-hydroxyphenyl-1-oxopropanoate (aq)[2] + H2O2 >> catechol + malonic acid [3]
[1] http://www.orgsyn.org/demo.aspx?prep=cv1p0235 (see Discussion; the extension from intermolecular to intramolecular is sort of a leap but
usually makes things easier anyway)
[2] http://www.sciencedirect.com/science/article/pii/S1386142506...
[3] http://orgsyn.org/demo.aspx?prep=CV1P0149 (it is an o-hydroxyketone at the end of the day)
-------
Also, the Baeyer-Villiger oxidation of levulinic acid gives 3-acetoxypropanoic acid. Levulinic acid in turn is produced from sucrose and HCl.
-------
EDIT 2:
Maybe you could make 1,3-dinitropropane by the Michael addition of nitromethane to niroethylene? Nitroethylene then is made from nitromethane and
formaldehyde!
Reduction with a variety of reagents (CrCl2 reportedly gives oximes IIRC) gives malondialdehyde dioxime, a precursor to malonic acid as well as many
other interesting compounds, including malononitrile.
-------
EDIT 3: last one i swear
ethyl chloroacetate + zinc + formaldehyde >> ethyl hydracrylate aka ethyl 3-hydroxypropanoate
(reformatsky reaction)
NB: with acetaldehyde this gives an ethoxide-free preparation of ethyl acetoacetate by oxidation
[Edited on 13-10-2016 by clearly_not_atara]
[Edited on 13-10-2016 by clearly_not_atara]
[Edited on 13-10-2016 by clearly_not_atara]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I was recently going through a dark corner of my lab when I found 4 samples of calcium salts prepared by the alkaline oxidation of malic acid in the
hope of producing malonic acid as described above. I decided to finally complete this work by analysing these precipitates. I decided to proceed by
adding just enough sulphuric acid to liberate the organic acid but since I didn't know exactly what compound I had and therefore how much acid it
would require I first had to analyse the Ca content. I did this by taking a small sub sample of about 0.2g accurately weighed out and calcining it in
a pre-weighed nickel crucible. The calcine was cooled and left in in a plastic box over ammonium carbonate for a few days to ensure conversion of any
calcium oxide to carbonate. The calcines were reheated to just 120 degrees in an oven to drive off any water and ammonia and then weighed. The weight
of CaCO3 remaining was then used to calculate the amount of sulphuric acid required. The weights seem rather higher than I had been expecting since I
had assumed that the precipitate consisted of a mixture of calcium malate, malonate 4 hydrate and oxalate dihydrate. The CaCO3 weights suggested that
they were anhydrous and even then there was too much calcium present. For one sample I dissolved to check the calcine by dissolving it in a little
dilute HCl, then diluting it to 250ml and titrating it as though it was hard water using a buffer, solochrome black as indicator and standard EDTA
solution (bought). This procedure confirmed that the calcine was essentially pure CaCO3.
One of the samples was too small to be worth treating but the other three were then reacted with the appropriate amount of 1M sulphuric acid which
resulted in copious evolution of a colourless odorless gas, certainly CO2. The slurry was warmed a little to ensure complete reaction and then chilled
overnight to allow the calcium sulphate to cystallise then evaporate down on a water bath in a shallow ceramic bowl to a thick solution and left to
crystallise. Only further calcium sulphate was recovered. No evidence of organic acids could be found in the residues, not even acetic acid from the
breakdown of malonic acid.
From this I conclude that the alkaline oxidation of Malic acid (more correctly malate salts) results not in malonic or even oxalic acid but instead
carbonate ions, chloroform and only organic acids that form soluble calcium salts such as formate or acetate. The method therefore is a complete
failure.
Just thought you ought to know! Back to the drawing board.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
There's a prep of 2-methylmalonic acid on Orgsyn which proceeds via 3-methyl-2-oxosuccinic acid. Apparently the ester of this is pyrolysed and
undergoes some kind of carbonyl-elimination rearrangement.
Starting from diethyl malate, oxidation with an anhydrous CrO3 synthon (such as PCC or something) should give oxaloacetic acid -- be aware that
alpha-ketoesters hydrolyse easily. Pyrolysis of this ester may, if the analogy holds, generate diethyl malonate.
I think it's worth a try, at least. There's also the possibility of somehow reducing alloxan to barbituric acid.
|
|
|
Niter of Potash
Harmless

Posts: 13
Registered: 28-12-2016
Member Is Offline
Mood: No Mood
|
|
Claisen condensation
My idea is to make malonic acid by mixed claisen condensation between ethyl formate and ethyl acetate.
That /product/ (i'm not good at naming compounds) would be ester on one side, and aldehyde on another. Oxidizing that aldehyde with HNO3, KMnO4 or
something silimar, sould yield monoester of malonic acid, or pure acid, if ester gets hydrolised.
Since I can't really get or make pure Na metal (I tried NurdRage's dioxane method, but since my stirrer is broken, I couldnt extract decent quantaty
of Na metal), I would probbaly use Na/MgO aggregate, mix that with dry EtOH, and filter, to get sodium ethoxide solution, and use that in reaction.
Sadly, I'm pretty buisy so I can't try this out, but can anyone confirm that it would work at all, or why not?
EDIT:
As soon as I posted reply, I relalised what could go wrong.
http://www.prepchem.com/synthesis-of-benzyl-benzoate/
Reactions similar to this could accure, and that would yield malonic acid - 3-hydroxypropionic acid ester.
That,however, is not a huge issue, as 3-hydroxypropionic acid could be further oxidised to form malonic acid.
Not a big deal, I guess, and this would only make extraction and purification steps a bit more complex
[Edited on 15-10-2017 by Niter of Potash]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Will diethyl carbonate undergo a cross claisen condenstion with ethyl acetate to give diethyl malonate directly? Or is this just wishful thinking?

|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In order to achieve the desired selectivity I would think you want to use diMethyl carbonate and tert-butyl acetate, although isopropyl acetate is
similar and much more OTC.
I think it's possible... yields may be complicated by the formation of acetoacetate.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Most of what is being proposed here is too far fetched for the typical hobby or home lab. Why not just buy diethyl malonate which is cheaply available
from perfume chemical suppliers. It is essentially OTC. The diester can serve as the basis for lots of chemistry including the preparation of the the
half-ester and malonic acid itself.
I realize folks like to speculate on all sorts of possible chemical reactions, but most of the time it's just easier to buy what you need. Do not mean
to offend, just my thoughts.
AvB
|
|
|
| Pages:
1
..
4
5
6
7
8
9 |