| Pages:
1
2 |
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Acetone from calcium acetate
btw i know i can just buy acetone from the hardware store.
i have dissolved some seashells in vinegar. which is the strangest things first time seeing seashells dissolve in an acid. Don't worry no crustations
weren't in them
and it should form calcium acetate. any ideas how the best way to make the acetone from there. Im guessing just heat it to decomposition to form
acetone then condense it with salt ice water bath. Also whats it decompose to acetone and what else is the calcium compound left over calcium oxide?
not sure what the decomposition reaction is for this.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Vinegar is less than 6% acetic acid and not all of it will have reacted.
But do the calculations on the basis of how much vinegar you used and work out your theoretical yield of calcium acetate.
The next step is to boil down the solution and dry your calcium acetate. Work out how much energy this requires!
Put this in to a suitable container and heat until pyrolysis takes place, yields are crap. Ten grams of calcium acetate will yield a few ml of impure
acetone under best conditions.
Redistil your acetone and work out how much it has cost you per ml.
Realise that you could have bought a litre of acetone for the cost of your vinegar, electricity, etc and still had change for a pint and a pizza 
|
|
|
Hexagon
Harmless

Posts: 45
Registered: 11-5-2010
Member Is Offline
Mood: Fanf*ckingtastic
|
|
Man he said he already knows he can get acetone for cheap out of the hardware store so that implies he is just doing it for fun or academical
pourposes...
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | what else is the calcium compound left over calcium oxide? |
Calcium carbonate. The idealized reaction is
Ca(CH3COO)2 -> CaCO3 + C3H6O
You need a fair bit of heat to drive the reaction; 350C at least and 450-500C would be better unless you have a lot of time to waste. I tried this
same reaction at around 250C and got nothing.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
that's interesting you end up with Calcium Carbonate again? calcium carbonate and acetic acid actually mine is more like 5% or more but more like
5.01% the way they short change it as vinegar has to be a certain %
i was interested on the old fashion way they made it.
[Edited on 2-5-2011 by symboom]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I like to try this reaction but i think:
-This is impossible to use glass rbf for this purpose(500C)(i should use stainless steel flask)
-Flask should be free of any oxygen otherwise acetone will fire and flask will explode.(i should use inert gas or i should use vacuum)
-Common burner cant provide 500C and i should use special electrical heater
-Produced Acetone should cool rapidly otherwise Self-condensation will occur(i should use inert gas for speedup acetone exit and cooling)
..
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
I suggest the use of pyrex test tubes if you have them. They allow much greater temperatures to be reached than RBF's. Calcium carbonate will go to
red hot if heated in one of those.
|
|
|
cyanureeves
National Hazard
   
Posts: 737
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
in spite of ScienceSquirrel saying it was not worth it, he did provide the instruction. 1 ml?you're better off selling pickled seashell by the
seashore . if i was ever stranded on a distant island and had three picks of company, i'd pick symboom for his raw ideas and ScienceSquirrel for know
how and a RBF.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by cyanureeves  | in spite of ScienceSquirrel saying it was not worth it, he did provide the instruction. 1 ml?you're better off selling pickled seashell by the
seashore . if i was ever stranded on a distant island and had three picks of company, i'd pick symboom for his raw ideas and ScienceSquirrel for know
how and a RBF. |
I don't know if your going to get very far without a burner, I'd say drop the RBF and get the burner, You could make all sorts of glassware with sand
and the burner--might be a bit opaque though..hahaha 
Further more, I don't think the synthesis is for any purpose than just to do it. Of course he/she could go out and buy a liter of Acetone for pocket
change, but that's not the point--Its for the science...and fun!
[Edited on 1-13-2012 by AirCowPeaCock]
BOLD
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
There are lots of things that are more fun, colourful and easier to synthesise.
Make some alums, double or complex salts.
Cheaper and more interesting than making a readily available organic solvent in low yield at high cost.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Agreed, it shouldn't be used if you have the intention of large-volume production but might just be nice for a prove-the-point experiment. You could
use some permangante and test for no discoloration with your acetone, as it is a ketone and won't be oxidised by the KMnO4 any further, resulting in
no oxidation or reduction of the KMnO4 and consequently no colour change.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
If he has KMnO4 at his disposal..I certainly don't, although I should. Maybe NaCO3.1.5H2O2? But that might create a peroxide group slowly
BOLD
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
I have tried this myself and uploaded a video to youtube but they blocked my video with a warning. I'm not sure why.
Yes, you do have to dry you calcium acetate. You can either leave it out in air or dry it with a heater. One way cost a lot of time and the other
costs an energy source.
You should heat at around 300 C. I used pyrex distillation setup.
White fumes form in the condenser but they conglomerate into drops.
The smell is weird. It smells like some kind of heavy hydrocarbon.
Once you finish, you end up with CaCO3 again.
You can drop more acid and see it fizz again.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by vmelkon  | I have tried this myself and uploaded a video to youtube but they blocked my video with a warning. I'm not sure why.
Yes, you do have to dry you calcium acetate. You can either leave it out in air or dry it with a heater. One way cost a lot of time and the other
costs an energy source.
You should heat at around 300 C. I used pyrex distillation setup.
White fumes form in the condenser but they conglomerate into drops.
The smell is weird. It smells like some kind of heavy hydrocarbon.
Once you finish, you end up with CaCO3 again.
You can drop more acid and see it fizz again. |
I Dont think at 300c thermal decompose occur.Can you explain your try( Ratio and your Yield)?
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
For heating, the first few times I used coal.
For the next few runs, I used 2 halogen bulbs in parallel (500 W and a 300 W). To my surprise, the halogen is does the job just as well.
I didn't take measurements since I didn't consider this experiment important 
I'm going to try again sometime.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I think barium acetate Pyrolysis should be more interesting(it decompose on 150C)
| Quote: |
When barium acetate is pyrolyzed it decomposes, forming acetone and barium oxide as pyrolysis products. Often, during the
heating, the acetone vapors catch fire and/or explode, and the further production of acetone is catalyzed by the barium oxide.
Melting point (decomposes): 150 °C
Jay A. Young
Chemical Consultant, Silver Spring, MD 20904-3105
J. Chem. Educ., 2006, 83 (3), p 380
DOI: 10.1021/ed083p380
Publication Date (Web): March 1, 2006
http://pubs.acs.org/doi/abs/10.1021/ed083p380
|
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Would need to evacuate the dist app though, but I suppose you would probably want to do that for Ca(AcO)<sub>2</sub>
According to Wiki Ca(AcO)<sub>2</sub> decomposes at 160 C
[Edited on 2-2-2012 by AirCowPeaCock]
BOLD
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Ca(AcO)2 decompose at 400-500C(i tried it.wiki is wrong)
The thermal decomposition of calcium, sodium, silver and copper(II) acetates
Journal of Thermal Analysis and Calorimetry
M. D. Judd, B. A. Plunkett and M. I. Pope
Volume 6, Number 5, 555-563,
DOI: 10.1007/BF01911560
http://www.springerlink.com/content/p637804801j11425/
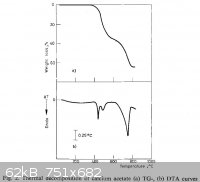
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
I frequently find wiki wrong on melting/boiling points, decomps, and solubility--but everything else seems to be in-order. Why the fuck would this
be, are assholes just pulling numbers out of their asshole? Not to long ago I caught wiki saying NaCl has a solubility of something along the lines
of 500 g / 100 ml
BOLD
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Interesting result by pyrolysis of Nickel Acetate(Diacetyl produced)
I like to know effect of vacuum in Nickel acetate Pyrolysis 

|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The thermal decomposition of sodium acetate forms acetone.
Adding sodium hydroxide to sodium acetate, then thermally decomposing, forms methane instead.
In both instances, sodium carbonate forms as a byproduct.
|
|
|
Aperturescience27
Harmless

Posts: 39
Registered: 5-4-2012
Member Is Offline
Mood: No Mood
|
|
What about using magnesium acetate? Seems simple enough, and according to Waffles SS's chart, it decomposes between 275 and 340 C. Still pretty high,
but better than calcium.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Aperturescience27  | | What about using magnesium acetate? Seems simple enough, and according to Waffles SS's chart, it decomposes between 275 and 340 C. Still pretty high,
but better than calcium. |
The hydrate of magnesium acetate loses water to become fully anhydrous above 150 °C. The anhydrous magnesium acetate then decomposes into MgO,
acetone, and CO2 at 275 to 340 °C.
"Kinetics of thermal decomposition of metal acetates", M. Afzal (Pakistan)
(note that the hydrate of magnesium nitrate decomposes into nitrogen oxides rather than giving up water)
[Edited on 7-6-2012 by AndersHoveland]
|
|
|
Aperturescience27
Harmless

Posts: 39
Registered: 5-4-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | Quote: Originally posted by Aperturescience27  | | What about using magnesium acetate? Seems simple enough, and according to Waffles SS's chart, it decomposes between 275 and 340 C. Still pretty high,
but better than calcium. |
The hydrate of magnesium acetate loses water to become fully anhydrous above 150 °C. The anhydrous magnesium acetate then decomposes into MgO,
acetone, and CO2 at 275 to 340 °C.
"Kinetics of thermal decomposition of metal acetates", M. Afzal (Pakistan)
(note that the hydrate of magnesium nitrate decomposes into nitrogen oxides rather than giving up water)
[Edited on 7-6-2012 by AndersHoveland] |
Are you agreeing with me? I don't see anything there that would be a problem. I think I might try this, actually.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
In 'The pyrolysis of carbon compounds' Hurd gives a temperature range of 330-360C for magnesium acetate. He also (on p482) lists the following yields
of acetone for pyrolysis of various acetate salts, which are from Krönig (Z. angew. Chem. 1924):
Lithium acetate: 100% (contradicted elsewhere in the text where he gives a 93% figure)
Barium 89%
Calcium 83% (but elsewhere 90-100% is claimed if inert gas sweeping is used)
Magnesium 76% (elsewhere 57% is claimed)
Manganese(II) 66%
Sodium 37% (elsewhere 50%)
Potassium 12%
Lead 87%
Hurd gives no complete list of temperature ranges for decomposition of all these salts, but it may be that Krönig's original paper has some such
information.
The less you bet, the more you lose when you win.
|
|
|
| Pages:
1
2 |