| Pages:
1
2
3 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Because the carbon-nitrogen triple bond is nearly as strong a the triple bond in diatomic nitrogen, it does not make much sense to incorporate cyano-
groups into explosives.
Such groups would also potentially create toxicity problems (unless they are in the form of a polymer)
The formation of N2 from --CN groups will not release much more energy than it took to break the carbon-nitrogen triple bond. Adding --CN groups to
the molecule is therefore somewhat analogous to attaching a canister of highly compressed nitrogen gas to the explosive. Yes, it will add to the
explosion, but it additional energy will be almost entirely entropic, which is to say that the nitrogen gas will just occupy a greater volume
when it is allowed to expand. This is in sharp contrast to tetrazoles, where the liberation of nitrogen corresponds to the formation of the strong
nitrogen-nitrogen triple bond, which lends plenty of energy (which is mostly utilized expanding the gas products).
[Edited on 20-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by AndersHoveland  |
Because the carbon-nitrogen triple bond is nearly as strong a the triple bond in diatomic nitrogen, it does not make much sense to incorporate cyano-
groups into explosives.
Such groups would also potentially create toxicity problems (unless they are in the form of a polymer)
The formation of N2 from --CN groups will not release much more energy than it took to break the carbon-nitrogen triple bond. Adding --CN groups to
the molecule is therefore somewhat analogous to attaching a canister of highly compressed nitrogen gas to the explosive. Yes, it will add to the
explosion, but it additional energy will be almost entirely entropic, which is to say that the nitrogen gas will just occupy a greater volume
when it is allowed to expand. This is in sharp contrast to tetrazoles, where the liberation of nitrogen corresponds to the formation of the strong
nitrogen-nitrogen triple bond, which lends plenty of energy (which is mostly utilized expanding the gas products).
|
How then do you account for the fact the following organic nitriles/cyanides are mostly if not all endothermic and displays very high flame
temperature?
H-C#C-H FT 3100°C (just put here as comparative example)
H-C#N FT over 3500°C
N#C-C#N FT over 4000°C
N#C-C#C-C#N FT over 4700°C
Carbon subnitride - dicyanoacetylen
Please note the high density and high heat of formation...
This and many other examples have brought me to the idea Hydrogen atom in a molecule is not very beneficial to explosive power while multiple bond do
contribute to higher densities and entrapped energy.
Simply compare cyclohexane and benzene over density and heat of combustion.
Also you are a bit preaching against your religion...because if we look further tetrazole, triazoles etc that you love so much are only the result of
a condensation between azido and cyano groups and the cyclisation into an aromatic like latice/fashion...
I'm quite sure that salts of H-C#C-C#N with AgNO3 or AgClO4 must be sensitive killers as compared to silver acetylide nitrate double salt...It will
display higher VODs and brisance.
Two other good challenge are H-C#C-C(NO2)3 (must be able to make sensitive very powerful initiators salts) and the probably very dense
(O2N)3C-C#C-C#C-C(NO2)3 ...
Yeah perfect OB, zero hydrogen and multiple bonds providing very high heat of detonation...blocked linear shape providing high density because no move
is allowed except external rotation of the nitroformyl moieties...
Franklyn has wel understood their potential as ingredient to increase inherent power.
The -C#N radical is listed in the explosophoric groups and that's for sure for a reason.
If someone has data on the heat of formation or of burning of
CH3-C#N (acetonitrile)
CH2(-C#N)2 (malononitrile - methylene dinitrile - methylene cyanide)
CH(-C#N)3 (cyanooform -nitriloform - tricyanomethane)
C(-C#N)4 (tetracyanomethane)
This would prove to all what Franklyn and I have the intuition of.
[Edited on 20-7-2011 by PHILOU Zrealone]
[Edited on 20-7-2011 by PHILOU Zrealone]
[Edited on 20-7-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I knew you were going to bring that up.
Strategies for achieving high flame temperature are not necessarily the same strategies that should be used for improving explosive performance,
although there is some overlap.
In my opinion, at least, the cyano groups in NΞC–CΞC–CΞN function more as non-hydrogen groups. If desiring to have a high flame
temperature, incorporation of hydrogen into the fuel molecule would not be ideal, as the water molecules that form will absorb a significant quantity
of heat. Not only does H2O absorb significant energy for its phase change (to steam), but more molecular vibrations are possible in the H2O molecule
than the N2 molecule, thus much of the heat is taken up vibrating the molecule instead of expanding the gas.
Such an argument could also be extended towards designing energetic compounds, but there are several reasons this can be problematic. Explosive
molecules without hydrogen atoms tend to be more sensitive, and less energetic per unit mass. Since hydrogen atoms have a small size, it can also be a
way to pack fuel into a molecule without taking up as much volume, potentially leading to higher energy density.
Combining carbon-carbon triple bonds and trinitromethyl groups is likely to lead to very high sensitivities, if not chemical instability. Although
trinitromethane itself is only about as sensitive as picric acid, mixtures with other organic compounds are much more dangerous. Frozen mixtures of
trinitromethane with 2-propanol (10%) explode when thawed. Mixtures with divinyl ketone, which contains unsaturated carbon bonds that are more
vulnerable to oxidation, can explode at 4°C ! Note that the hydrogen atom in trinitromethane greatly adds to thermal stability, without it the
trinitromethyl group is much more liable to partially decompose giving off oxides of nitrogen in storage. The oxides of nitrogen will spontaneously
react with the carbon-carbon triple bond, likely leading to significant degredation problems, or even danger of a spontaneous run-away reaction that
could initiate detonation in storage (like trinitroanaline) ! Although trinitromethane will oxidize Fe+2 to Fe+3 (indicating it would probably be
incompatible with alkynes), the trinitromethyl group (attached to another carbon atom) is in some ways much more inert (for example it is not
vulnerable to acid hydrolysis). However, trinitromethyl groups will nevertheless slowly decompose in hot water. Yes, no doubt such compounds would be
extremely powerful, but the question is safety and stability.
quote by PHILOU Zrealone: "other examples have brought me to the idea Hydrogen atom in a molecule is not very beneficial to
explosive power while multiple bond do contribute to higher densities and entrapped energy. Simply compare cyclohexane and benzene over density and
heat of combustion."
This is not really the best example. Benzene probably has a higher density because the molecules are planar, and there is more intermolecular
attraction between the molecules because of the unique delocalized nature, in some respects almost similar to metallic bonding. Cyclohexane contains
more potential energy of combustion per unit mass than benzene, not only because of the lightweight hydrogen, but also because the hexagonal aromatic
ring in benzene is so stable.
quote by PHILOU Zrealone: "Also you are a bit preaching against your religion...because if we look further tetrazole, triazoles
etc that you love so much are only the result of a condensation between azido and cyano groups and the cyclisation into an aromatic..."
It is true that I have "preached" against cyano and azido groups; however, when cyclized together into a tetrazole, the properties change. Much of the
energetic nature of the azido group is conserved while the sensitivity is markedly reduced. The carbon-nitrogen triple bond turns into much weaker
"one single and one double" carbon-nitrogen bond.
The pentagonal ring provides some ring strain, while the aromatic nature adds stability. Tetrazoles also contain an NH group within the ring that is
electron-donating, adding stability especially when an electron-withdrawing nitro or nitrimino group is present. This NH group, containing only one
nitrogen-hydrogen bond, is more energetic than the usual amine NH2 group often used to stabilize aromatic explosives. The NH group in triazole rings
is also much more resistant to oxidation, potentially reducing the complexity of extreme nitrations/oxidations during synthesis.
As for high flame temperatures, you may be interested in the cyclic alkynes, which consist of large rings of carbon atoms alternating between single
and triple bonds. It is difficult to find anything online about them, but I remember reading of their preparation in a chemistry journal from the
1980's.
"Cyclo[18] carbon is a highly reactive compound containing an 18-membered ring with alternating single and triple bonds." (botton of p3) http://www.uiowa.edu/~c004121/notes/ch11_3.pdf
http://www.sciencemag.org/content/245/4922/1088.abstract
http://en.wikipedia.org/wiki/Cyclocarbon
[Edited on 20-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I want to mention my strong belief in the promise of using 1,2,3-triazoles in the design of future energetic compounds. Unfortunately, the synthesis
of 1,2,3-triazoles is fairly complex (much more difficult than 1,2,4-triazoles) and there is not much information available about an easy synthesis,
or the properties of its derivitives.
1,2,3-triazole itself is surprisingly relatively insensitive, and can be safely shipped. 1,2,3-triazole dervitives are also more thermally stable and
resistant to hydrolysis than 1,2,4-triazole. 1,2,3-triazole explosives would also be expected to be much more powerful than either 1,2,4-triazoles or
1,2,4,6-tetrazines. 1,2,4,6-tetrazine (C2H2N4), although containing one more nitrogen atom than triazole (C2N3H3), contains much more carbon-nitrogen
bonds (in addition the hexagonal aromatic ring is extremely stable), such that it is not really any more energetic than plain hydrazine.
The 1,2,3-triazole ring is very much comparable to tetrazole, except the extra carbon atom allows two energetic groups to be attached
to the ring, instead of just one.
[Edited on 20-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
(already posted a link to the below, but wanted to actually include it in this forum)
I have designed some potential target molecules that may eventually lead to new explosives which are both safer and more powerful. While the synthesis
is probably beyond most of the readers here, I nevertheless wanted to share these ideas with the forum.
4-nitro,5,6-triazolo-1,2,3-triazine-2,7-N-dioxide (NTTO), C3N7HO4
may likely be as powerful as octonitrocubane, while being less sensitive than HMX
https://sites.google.com/site/ecpreparation/ntto
Furoxanyltriazole oxide (FTO), C2N5HO3
could potentially exceed HMX in performance, while being a safer explosive, being significantly more resistant to impact.
https://sites.google.com/site/energeticscribble/furoxanyltri...
https://sites.google.com/site/ecpreparation/fto
TEAN, C6H9N7O6
a molecule with some similarities to RDX, but with a caged structure. Like another experimental energetic compound, TEX, it should be less sensitive,
and somewhat more powerful than RDX. The pressence of a tertiary amine on the molecule opens up the possibilities of even more powerful derivitives,
either an N-oxide, or possibly even nitrate salts.
https://sites.google.com/site/ecpreparation/tean
N,N’-azoxy-4,4’-bis[5-nitro-1,2,3-triazole]-1-oxide
a bridged triazole compound that would be more powerful than HMX, and possibly somewhat more resistant to impact. It should be fairly easy to prepare
from energetic precursors that are already well described in recent publications.
https://sites.google.com/site/ecpreparation/oxidizing-antz
4,8-diamino-3,7-dinitro-1,2,5,6-tetraazobicyclo octene (ANTAZBO),
or alternatively the central adjoining double pentagonal-ringed frame could be described as
pyrrolo[2,1-c]-1,2,3-triazole (PT), or even more accurately as
1,2,5,6-tetrazocyclooctatetraene.
The molecular structure can be described as C4N4(NH2)2(NO2)2
This molecule is based on a frame with a similar structure to 3,6-Dinitropyrazolo[4,3-c]pyrazole (DNPP), which is an energetic compound that has
already be prepared adescribed in literature. Unlike DNPP, however, the carbons in the center have been switched with nitrogens from the outside,
allowing four side groups to be bonded to carbon atoms, instead of only two. This compound should be relatively insensitive, white at the same time
having excellent performance, approaching the power of HMX. With 3 nitro groups instead of two, and one less amino group, it would likely be even more
powerful than HMX, although it would then be significantly more sensitive, lsoing some of its resistance to impact.
https://sites.google.com/site/energeticscribble/1-2-5-6-azob...
5,5,6,6-tetranitro-2,3-diazobicyclo[2.1,1]hexane (NDZBH), C4H2N6O8
a caged molecule, similar to 1,1,3,3-tetranitrocyclobutane, but with a diazo bridge that adds both more nitrogen and a large ammount of molecular
strain. you can view the skeletal structure (without the four nitro groups) of 2,3-diazobicyclo[2.1,1]hexane here http://energetic.proboards.com/index.cgi?action=downloadatta...
For a comparison, here is some information about 1,1,3,3-tetranitrocyclobutane: estimated detonation pressure between 372-400 kbar, density 1.83 g/mL,
melting point 165 degC (not considered melt-castable, significant decomposition), acronym TNCB, performance somewhat better than HMX. Like TNAZ, which
has been thoroughly studied, TNCB would be expected to show good thermal stability, despite the geminal nitro groups, because of the ring strain
preventing ionization and concurrent carbon-carbon double bonds. TNCB is almost certainly less sensitive than HMX, as TNAZ is fairly insensitive. The
diazo bridge, --N=N--, in DNZBH, however would add sensitivity, so it is difficult to speculate on how it would compare to HMX.
It is quite probable that NDZBH would be comparable to octonitrocubane in power.
[I]note about structure[/I]: despite the nitro groups in the 5- and 6- positions, they are not vicinal since the carbons in the
"5-" and "6-" positions in the cage's nomenclature are [U]not[/U] bonded to eachother. In other words, there are two geminal nitro groups on each
carbon in the square ring that is not bonded to the other two nitrogen atoms.
https://sites.google.com/site/ecpreparation/2-3-diazobicyclo...
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
simply RED
Hazard to Others
  
Posts: 206
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
An unsolvable problem presents the fact that the oxidizer groups -NO2, -O-NO2, =N-NO2 have electron withdraw effect such as the good fuel groups -CN,
-C3bondsC-. This makes a molecule having both groups extremely unstable.
For example dinitroacetylene if existed would be unstable.
[Edited on 22-7-2011 by simply RED]
When logic and proportion have fallen sloppy dead...
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by simply RED  | An unsolvable problem presents the fact that the oxidizer groups –NO2, –O–NO2, =N-NO2 have electron withdraw effect such as the good fuel groups
–CN, –CΞC–. This makes a molecule having both groups extremely unstable.
For example dinitroacetylene if existed would be unstable.
|
I would not say a molecule is unstable because it has all electron-withdrawing groups, just that oxidizing groups in energetic molecules tend
to make the compound more sensitive if there is not some other group that can be electron donating towards it. Even with the nitramine explosive RDX,
the inner nitrogen atoms are probably somewhat electron donating towards the nitro groups. This electron donating effect would be expected to be
reduced if the methylene groups (-CH2-) only had one hydrogen on them, or if a methylene group were replaced by a carbonyl group -C(=O)-, as for
example in "keto-RDX". The nitrimino group =N-NO2 is typically much less unstable without the electron donation effect. The nitrimino group could
actually be more "acurately" written as
–N=NO2[-], and thus it can be seen the strong similarity of the group to a nitrate anion. I really have no idea why dinitroacetylene has so far
evaded synthesis, or what its sensitivity would be. It may be possible that the molecule would spontaneously polymerize because of the additional
presence of nitro groups.
The following may be helpful if wishing to type molecular structures:
Ξ greek letter "xi"
— "em dash"
– "en dash"
- hyphen
If you cannot figure out how to type the symbols using your key board, you can always use the "copy" and "paste" method.
[Edited on 22-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@AH, Confession is good for the soul. So tell us if you have ever synthesized RDX,
much less any of the more exotic energetic theoretical materials whose technical challenge you estimate is beyond the ability of those you regard as
your students here at the SM forum, the economic impracticality of some of these proposed materials for your envisioned powder monkeys of the future
notwithstanding.
Name that tune while you are at it
http://www.youtube.com/watch?v=5WsRuzWsZ1s
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Why should he not have prepared RDX - dropping hexamine into nitric acid isn't exactly difficult?
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Bridged Heterocyclium Di-Cationic closo-Icosahedral ....
Have stumbled upon this. There dobe a SL of papers
published every year on new explosives &c.
Accession Number : ADA521182
Title : Bridged Heterocyclium Di-Cationic closo-Icosahedral
Perfluoroborane, Borane, and Carborane Salts via Aqueous, Open-
Air Benchtop Synthesis (Preprint)
Descriptive Note : Journal article
Corporate Author : AIR FORCE RESEARCH LAB EDWARDS AFB CA
PROPULSION DIRECTORATE
Personal Author(s) : Shackelford, Scott A. ; Belletire, John L. ;
Boatz, Jerry A. ; Schneider, Stefan ; Wheaton, Amanda K. ;
Wight, Brett A. ; Ammon, Herman L. ; Peryshkov, Dmitry V. ;
Strauss, Steven H.
Handle / proxy Url : http://handle.dtic.mil/100.2/ADA521182
Report Date : 11 MAR 2010
Pagination or Media Count : 30
Abstract : Thirteen unreported bridged triazolium and imidazolium
di-cationic salts, that uniquely pair closo-icosahedral
perfluoroborane [B12F12](exp 2-), borane [B12H12](exp 2-), or
carborane [CB11H12](exp -) anionic species with unsaturated
bridged heterocyclium di-cations, were synthesized in water using
an open-air benchtop method. This considerably extends the
scope of a reported aqueous synthesis of binary
[Heterocyclium]2[B12H12] and [Heterocyclium][CB11H12] salts.
Also, the one-step preparation of five new precursor bridged
heterocyclium di-cationic di-halide salts using conventional
procedures, and in one case a microwave-assisted procedure, is described.
Descriptors : *SALTS, *BORANES, MOLECULAR STRUCTURE,
AQUEOUS SOLUTIONS, CARBORANES, SYNTHESIS(CHEMISTRY),
CRYSTAL STRUCTURE
Subject Categories : ORGANIC CHEMISTRY PHYSICAL CHEMISTRY
Compared to neutral organic compounds, heterocyclic
salts enhance the flexibility to attain rational structural
design, and resultant predicted ingredient properties, that
can permit a tailorable behavioral response.1,2 Tailoring
thermal initiation of heterocyclium borane and di-nitrate
salts to an air-sustained combustion is one example,2 as is
explained by a current initiation sensitivity concept.3
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Heterocyclic Salt Synthesis and Rational Properties Tailoring
Accession Number : ADA513619
Title : Heterocyclic Salt Synthesis and Rational Properties Tailoring (PREPRINT)
Descriptive Note : Journal article preprint
Corporate Author : AIR FORCE RESEARCH LAB EDWARDS AFB CA
PROPULSION DIRECTORATE
Personal Author(s) : Shackelford, Scott A. ; Belletire, John L.
Handle / proxy Url :
Report Date : 23 JUN 2009
Pagination or Media Count : 23
Abstract : Chemical structure determines the inherent properties
displayed by a given compound, and these properties, in turn,
produce a specific performance behavior. Rationally designing
chemical structure to predictably modify compound properties,
such that performance behavior can be tailored in a controlled
manner, defines the objective of a pertinent synthesis effort.
Achieving this objective by introducing structural alterations in a
neutral covalent compound offers only one approach for resultant
properties modification. Heterocyclic salts significantly enhance the
flexibility for achieving properties modification via three strategic
approaches: (1) compositionally pairing various cation structural
classes with a number of anion structural classes, (2)
systematically altering the structure of the cation; and, (3)
systematically altering the structure of the anion. To illustrate this
premise, four general synthesis methods to synthesize heterocyclic
salts, including several new binary heterocyclium icosahedral
closo-borane and closo-carborane salts, first are outlined.
Secondly, properties modification approaches of neutral covalent compounds are then compared with those approaches available for various heterocyclic
salts. Lastly, a key example, using three
unsaturated bridged heterocyclium di-cation salts, demonstrates
how rational structure design, and its effect on resultant
predictable properties modification, produces tailored performance
behavior to reach the thermochemical initiation threshold needed
for combustion. This is achieved with predictable properties
modifications that increase salt energy content, or that accelerate
the reaction kinetics of the thermochemical initiation process.
Descriptors : *REACTION KINETICS, *HETEROCYCLIC
COMPOUNDS, *PROPELLANTS, *ENERGETIC PROPERTIES, *SALTS,
*MODIFICATION, *MOLECULAR STRUCTURE,
*SYNTHESIS(CHEMISTRY), BEHAVIOR, ANIONS, CARBORANES,
THERMOCHEMISTRY, COMBUSTION, ENERGY, COVALENT BONDS,
THRESHOLD EFFECTS, NEUTRAL, BORANES, CATIONS,
PREDICTIONS
Subject Categories : ORGANIC CHEMISTRY
PHYSICAL CHEMISTRY
ROCKET PROPELLANTS
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Heterocyclic Salt Synthesis and Rational Properties Tailoring
I have to do a better job of tying my shoe laces in the
future I keep stumbling across things.
Accession Number : ADA513619
Title : Heterocyclic Salt Synthesis and Rational Properties Tailoring (PREPRINT)
Descriptive Note : Journal article preprint
Corporate Author : AIR FORCE RESEARCH LAB EDWARDS AFB CA PROPULSION DIRECTORATE
Personal Author(s) : Shackelford, Scott A. ; Belletire, John L.
Handle / proxy Url : http://handle.dtic.mil/100.2/ADA513619
Report Date : 23 JUN 2009
Pagination or Media Count : 23
Abstract : Chemical structure determines the inherent properties
displayed by a given compound, and these properties, in turn,
produce a specific performance behavior. Rationally designing
chemical structure to predictably modify compound properties,
such that performance behavior can be tailored in a controlled
manner, defines the objective of a pertinent synthesis effort.
Achieving this objective by introducing structural alterations in a
neutral covalent compound offers only one approach for resultant
properties modification. Heterocyclic salts significantly enhance the
flexibility for achieving properties modification via three strategic
approaches: (1) compositionally pairing various cation structural
classes with a number of anion structural classes, (2)
systematically altering the structure of the cation; and, (3)
systematically altering the structure of the anion. To illustrate this
premise, four general synthesis methods to synthesize heterocyclic
salts, including several new binary heterocyclium icosahedral
closo-borane and closo-carborane salts, first are outlined.
Secondly, properties modification approaches of neutral covalent
compounds are then compared with those approaches available for
various heterocyclic salts. Lastly, a key example, using three
unsaturated bridged heterocyclium di-cation salts, demonstrates
how rational structure design, and its effect on resultant
predictable properties modification, produces tailored performance
behavior to reach the thermochemical initiation threshold needed
for combustion. This is achieved with predictable properties
modifications that increase salt energy content, or that accelerate
the reaction kinetics of the thermochemical initiation process.
Descriptors : *REACTION KINETICS, *HETEROCYCLIC
COMPOUNDS, *PROPELLANTS, *ENERGETIC PROPERTIES, *SALTS,
*MODIFICATION, *MOLECULAR STRUCTURE,
*SYNTHESIS(CHEMISTRY), BEHAVIOR, ANIONS, CARBORANES,
THERMOCHEMISTRY, COMBUSTION, ENERGY, COVALENT BONDS,
THRESHOLD EFFECTS, NEUTRAL, BORANES, CATIONS,
PREDICTIONS
Subject Categories : ORGANIC CHEMISTRY
PHYSICAL CHEMISTRY
ROCKET PROPELLANTS
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
1,3,5,5-Tetranitrohexahydropyrimidine (DNNC)
Elucidation of such mechanistic features should aid in the
structural design of new high energy compounds with improved
thermochemical properties.
Accession Number : ADA491044
Title : Liquid State Thermochemical Decomposition of Neat
1,3,5,5-Tetranitrohexahydropyrimidine (DNNC) and its DNNC-d2,
DNNC-d4, DNNC-d6 Structural Isotopomers: Mechanistic Entrance
into the DNNC Molecule
Descriptive Note : Journal article
Corporate Author : AIR FORCE RESEARCH LAB EDWARDS AFB CA
PROPULSION DIRECTORATE
Personal Author(s) : Shackelford, S. A. ; Menapace, J. A. ; Goldman, J. F.
Handle / proxy Url : http://handle.dtic.mil/100.2/ADA491044
Report Date : 25 NOV 2007
Pagination or Media Count : 19
Abstract : Global kinetics for the liquid state thermochemical
decomposition of neat 1,3,5,5-tetranitrohexahydropyrimidine
(DNNC), perdeuterio-labeled DNNC-d6, and partially deuterium-
labeled DNNC-d2 and DNNC-d4 isotopomers were obtained by
isothermal differential scanning calorimetry (IDSC). Molecular
kinetic deuterium isotope effect (KDIE) values obtained with DNNC
and DNNC-d6 from 174 to 194-deg C revealed that C-H bond
rupture regulates both an endothermic catalytic initiation and the
exothermic propagation of the liquid thermochemical
decomposition process. Using IDSC-based KDIE comparisons with
the DNNC-d2, DNNC-d4, and DNNC-d6 isotopomers, a more
detailed chemical structure/mechanistic relationship emerged by
entering the interior of the DNNC molecule. Here structural kinetic
KDIE results showed the rate-controlling C-H bond rupture has its
origin at the non-equivalent C-2 methylene group sandwiched
between the two nitrated DNNC nitrogen ring atoms, versus at the
chemically equivalent C-4 and C-6 methylene ring positions
located elsewhere in the DNNC molecule. Elucidation of such
mechanistic features should aid in the structural design of new high
energy compounds with improved thermochemical properties. A
170.0 kJ/mol activation energy appeared for the endothermic
induction period, and a lower 104.2 kJ/mol activation energy was
determined for the exothermic acceleratory portion of the DNNC
decomposition process. The global liquid and solid state
thermochemical decomposition processes for DNNC are compared.
Descriptors : *KINETICS, *DECOMPOSITION,
*THERMOCHEMISTRY, ISOTOPES, MOLECULAR STRUCTURE,
DEUTERIUM, DIFFERENTIAL SCANNING CALORIMETRY,
PYRIMIDINES, REPRINTS, ISOTHERMS, ENDOTHERMIC
REACTIONS, NITRO RADICALS, LIQUIDS, EXOTHERMIC
REACTIONS
Subject Categories : PHYSICAL CHEMISTRY MECHANICS
djh
----
And thus ends today stumbling, I will now go forth into my 180+
acre wood lot to there wander aimlessly while — mumbling
incoherently, gesticulating wildly and drooling.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Role of Thermochemical Decomposition in Energetic Material Initiation Sensitivity and Explosive Performance
Accession Number : ADA468050
Title : Role of Thermochemical Decomposition in Energetic
Material Initiation Sensitivity and Explosive Performance
Descriptive Note : Conference paper (preprint)
Corporate Author : AIR FORCE RESEARCH LAB EDWARDS AFB CA
Personal Author(s) : Shackelford, Scott A.
Handle / proxy Url : http://handle.dtic.mil/100.2/ADA468050
Report Date : 05 FEB 2007
Pagination or Media Count : 31
Abstract : Catastrophic initiation of an energetic material consists
of a complex, interactive, sequential train of mechanistic
mechanical, physical, and chemical processes which occur over a
finite time period and proceed from macroscopic into sub-
microscopic composition levels (bulk > crystalline > molecular >
atomic). Initiation results when these processes proceed at a rate
which generates sufficient energy (heat) to reach a threshold stage
within this finite time period. Thus, the rate at which these
mechanistic processes occur defines initiation sensitivity and
affects performance. Thermochemical decomposition processes
regulate the rate at which heat energy is released at the molecular
level, and therefore to some extent, control energetic material
initiation sensitivity and performance characteristics. Kinetic
deuterium isotope effect (KDIE) data, obtained during ambient
pressure thermochemical decomposition process, identifies the
mechanistic rate-controlling bond rupture which ultimately
regulates the energy release rate of a given energetic material.
This same rate-controlling bond rupture also appears as a
significant rate-limiting feature in higher order deflagration,
combustion, and explosion phenomena. The effect the KDIE-
determined rate-controlling bond rupture exerts on initiation
sensitivity, and its potential influence in combustion and explosion
performance is delineated.
Descriptors : *SENSITIVITY, *EXPLOSIVES,
*THERMOCHEMISTRY, *DECOMPOSITION, *ENERGETIC
PROPERTIES, SYMPOSIA, ISOTOPE EFFECT, KINETICS,
DEUTERIUM, MATERIALS, RATES
Subject Categories : PHYSICAL CHEMISTRY AMMUNITION AND
EXPLOSIVES
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
this is just more conjecture, but wanted to discuss the pyrrolo[2,1-c]-1,2,3-triazole based molecule ("ANTAZBO"), which was proposed in my previous
post in this thread. the attached picture shows some of the different resonance structures, which would have a strong stabilizing effect on the rings
and nitro groups. This is indicative that the compound would have relatively low sensitivity. The electron donating effect also is one of the reasons
for the insensitive nature of triaminotrinitrobenzene, which is the chemical explosive used in nuclear weapons for this same reason. Fox-7 is another
example were the electron-donating effect is key to the molecule's stability.
(B) should have low sensitivity, with excellent performance
(C) even with three nitro groups on the molecule, the compound would liklely not be too sensitive
(F) in this resonance structure both adjacent nitro groups have extra electrons, which greatly increases stability, otherwise two adjacent nitro group
on an aromatic ring typically increases sensitivity of the compound
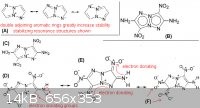
A clearer version of the same picture can be seen here:
https://3462015841141507561-a-1802744773732722657-s-sites.go...
also wanted to include the idea for a potential synthesis again,
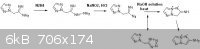
This would be somewhat similar to the procedure that has already been done by
R.A. Carboni, J.C. Kauer, J. American Chem. Society, volume 89, p2633, (1967).
although their reaction would not have been complicated by equilibrium with the tetrazole.
[Edited on 5-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quoted from Anders Hoveland
I want to mention my strong belief in the promise of using 1,2,3-triazoles in the design of future energetic compounds. Unfortunately, the synthesis
of 1,2,3-triazoles is fairly complex (much more difficult than 1,2,4-triazoles) and there is not much information available about an easy synthesis,
or the properties of its derivitives.
End of Quote
A simple way to 1,2,3 triazoles is via ortho-diaminobenzene and nitrous acid!
(the C=C being part of an aromatic ring!)
NH2-C=C-NH2 + HO-N=O --> O=N-NH-C=C-NH2 + H2O
O=N-NH-C=C-NH2 <--> HO-N=N-C=C-NH2
HO-N=N-C=C-NH2 --> cyclo(-N=N-C=C-NH-)
[Edited on 23-9-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
A simple way to 1,2,3 triazoles is via ortho-diaminobenzene and nitrous acid!
(the C=C being part of an aromatic ring!)
NH2-C=C-NH2 + HO-N=O --> O=N-NH-C=C-NH2 + H2O
O=N-NH-C=C-NH2 <--> HO-N=N-C=C-NH2
HO-N=N-C=C-NH2 --> cyclo(-N=N-C=C-NH-)
|
Yes, but then you are stuck with an unwieldy benzene ring to your triazole. Not to say that benzene rings are inherently bad, but because benzene has
so much carbon, it is hard to make benzene derivitives compete with the newer more powerful explosives.
I know this reaction is correct, as I have seen it before, but can you share the specific reference source with us?
[Edited on 24-9-2011 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Back to Basics
Molecular Structure & Performance of High Explosives.pdf
Attachment: Molecular Structure & Performance of High Explosives.pdf (488kB)
This file has been downloaded 840 times
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
The Wikipedia article on benzotriazole gives the following reference for the synthesis:
Robert A. Smiley “Phenylene- and Toluenediamines” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
[Edited on 19-12-2011 by Lambda-Eyde]
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
killswitch
Hazard to Others
  
Posts: 209
Registered: 8-7-2011
Location: is a relative concept
Member Is Offline
Mood: No Mood
|
|
This might seem a little weird or off topic, but has anyone tried feeding explosive precursors to various microorganisms to see if they shit out
anything useful? There must be some chemosynthetic organism somewhere that makes an energy profit from oxidation of amines. Perhaps they
carry an enzyme or two with interesting properties.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ killswitch
http://www.sciencemadness.org/talk/viewthread.php?tid=10976#...
http://www.sciencemadness.org/talk/viewthread.php?tid=4380&a...
________________________________________________________
HCN is endothermic having a Heat of Formation of + 32.3 Kcal/ mol , ~ +1196.3 Kcal/ Kg
Heat of formation , + 32.3 for HCN ( g ) , - 57.8 for H2O ( g ) , - 94 for CO2
. . . . . . . . . . . . . . . . . 4 HCN + 5 O2 . .=>. . 2 H2O . . . . . + . . . . . 4 CO2 . . . . + . . . . 2 N2
. . . . . . . . . . . . . . . . . .+129.2 . . . . . . . . . . . . - 115.6 . . . . . . . . . . . .- 376
The heat of reaction for the balanced equation as shown is - 620.8 kilocalories
Dividing that by the coeficient 4 , the heat of combustion of HCN works out to -155.2 kilocalories / mol , ~ - 5748 Kcal/ Kg
The weight of HCN + oxygen constituents is , 4(27) + 10(16) = 268
1000 x ( 620.8 ÷ 268 ) = 2 3 1 6 Kcal/ Kg for the oxygen balanced mixture
For comparison the energy obtained from NH4NO3 + 2 Al => Al2O3 + N2 + 2 H2
is ~ - 2900 Kcal/ Kg , twice that of glyceryl trinitrate
_______________________________________
What can be inferred from the above excercise is that the energy of a reaction is
derived from the fuel portion , the oxidizer unless it has some energy endothermically,
is just as much dead weght as unburned fuel. H2O2 has a density of 1.432 gm/cc in
98% strength, while liquid O2 at normal boiling point has a density of 1.142 gm/cc. .
Even with hydrogen included, H2O2 has 18 % more oxygen per unit volume than
liquid O2 ! Extra energy is also additionally provided by it's endothermicity. The low
output of ANFO ( NH4NO3 + fuel oil ) is directly attributable to the low fuel content
~ 6 % of the weight. This also illustrates why Sprengel type explosive mixtures have
greater energy since the fuel is not partially " burned " by a bond attaching the oxidizer
functional group. The Heat of Formation of Triazine the trimer of HCN , is + 41 ,
markedly less than the sum of the three individual HCN constituents , which
demonstrates the loss which occurs from bonding. A stable moiety made up of X
number of HCN constituents + Oxygen in the mol ratio of 2C 2H 2N 5O will obtain
close to maximum possible energy output. Glycol Dinitrate C2H4N2O6 for example
approximates the stated ratio and achieves ~ 1500 Kcal/ Kg. Nitrocarbons devoid of
hydrogen achieve the greatest energy product , ~ 1800 Kcal/ Kg for Hexanitrobenzene.
The molecular properties particularly density , as well as number of gas products will
determine the detonation characteristics of the explosive.
Having highly endothermic fuel moieties attached with oxidizing functional groups
leads to inevitable sensitivity if not actual instability. In the second half of this post
www.sciencemadness.org/talk/viewthread.php?tid=1970&page...
I outlined a method of circumventing that by having the groups separated in the
form of their ionic salts , then crystallized together.
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The CΞN triple bond is significantly stronger than than the C=N double bond. If one wishes to incorporate nitrogen into the molecule, it is
preferable not to have the nitrogen atom already triple-bonded.
Quote: Originally posted by franklyn  | An interesting question that remains to be resolved is whether it is better
on the whole to pursue zero oxygen balance or forego this in favor of producing a somewhat greater number of molecular detonation products.
|
Interesting and important question. My opinion about this is that explosives that decompose into CO2 tend to be somewhat more powerful than
when the decomposition product is mostly CO, but the difference does not seem to be big. Of course it depends very much on how the nitrogen is bonded
to begin with, but the formation of N2 seems to be potentially better than either CO or CO2, but not extremely so. Considering the sensitivity versus
power tradeoffs, I am rather partial to explosives that contain a mix of CO and CO2 in their decomposition products, with the nitrogen in the original
molecule mostly being used to hold oxygen, and optionally with one NH or NH2 group to serve as an electron donor (resulting in a reduction of
sensitivity) and making the molecule more polar (also decreasing sensitivity and typically allowing closer molecular packing, increasing density).
[Edited on 28-12-2011 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ AndersHoveland
| Quote: | The CΞN triple bond is significantly stronger than than the C=N double bond.
If one wishes to incorporate nitrogen into the molecule, it is preferable not
to have the nitrogen atom already triple-bonded. |
You still don't get it , even after PHILOU Zrealone exhaustively explained it.
The falacy of your assertion cannot be more stark than seen in the example I gave
above of the heat of formation of 3 HCN molecules relative to the heat of formation
of one trimer of the same three. 3 (+ 32) against 1 (+ 41).

Bond energies as referenced above
CΞN , 891
C=N , 615
C=C , 611
C -C , 347
C -N , 293
N -N , 159
Taking the bonding of two adjacent atoms in isolation of the whole molecule
does not characterize it.
Please observe your dangling particles , -CΞN , =C=N-
There is no possible arrangement of bonds other than CΞN that does not
produce a higher enthalpy !
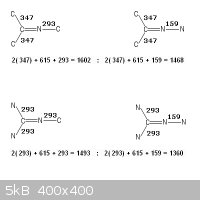
N=C=N -C , (615 + 615 + 293) = 1523
C=C=N -C , (611 + 615 + 293) = 1519
N=C=N -N , (615 + 615 + 159) = 1389
C=C=N -N , (611 + 615 + 159) = 1385
C -CΞN , (347 + 891) = 1238
N -CΞN , (293 + 891) = 1184
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Look at the attached diagram. (Obviously we are disregarding bond strain. It is only a diagram. The only reason it is in a square is to avoid making
it overcomplicated  ) )
In both arrangements, the two carbon atoms and two nitrogen atoms are all completely bonded with eachother. The net bonding energy of cyanogen, which
contains the carbon-nitrogen triple bonds, is 2129. But the net bonding energy is lower in the lower configuration, at exactly 2000. Lower
bond energy means the initial bonds are easier to break, so conversely the explosive will be more energetic.
Am I misunderstanding something?
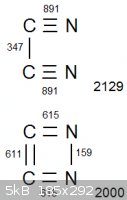
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Okay , leave it to you to tender an example to bolster your contention.
Take as an example another hypothetical molecule NΞC-NO2
essentially replacing the H in HCN with NO2. Just as in the example
of the trimer of 3 HCN , the trimer of 3 NΞC-NO2 , Trinitrotriazine
forms at greater enthalpy ( but not by much).
Bond strain is mostly pertinent to carbon to carbon bonding since a
strained nitrogen to nitrogen bond is easily broken , in fact isomerism
will impede their stable formation and defeat such arrangement reducing
the molecules energy , which will otherwise be too sensitive for practical
application. Perhaps you are on to something regarding the new mostly
nitrogen species being investigated made up with some carbon atoms,
clearly in such case your observation is justified , but may not provide
all that much advantage after all compared to more familiar formations.
See => Tris Tetrazolo Triazine < = > Cyanuric Triazide
http://www.sciencemadness.org/talk/viewthread.php?tid=4094#p...
Resonant bonding is perhaps why furoxans are so energetic despite
the low oxygen balance. Observing also that the achieved density greatly
factors into the equation , such as in NTTO and some variations proposed
by you elsewhere.
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
Synthesis of New High-Oxygen Carriers & Ditetrazinetetroxide (DTTO)
www.dtic.mil/dtic/tr/fulltext/u2/a513104.pdf
.
|
|
|
| Pages:
1
2
3 |
|