| Pages:
1
2
3 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Of course, 1,3,5-triazine is not an ideal scaffold for explosive molecules. The ring is aromatic, and that makes the bonding much stronger. In
addition, better to have more nitrogen-nitrogen bonds (double or single), because they are much weaker. And of course, trinitrotriazine is merely a
theoretical molecule. The compound has never been isolated, and would not be expected to be chemically stable.
The good thing about resonance is that it generally results in reduced sensitivity, which is an important consideration when trying to stick on as
much nitrogen or nitro groups as possible. Resonance also can affect chemical properties, typically making a ring scaffold easier to chemically alter.
[Edited on 28-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by killswitch  | | This might seem a little weird or off topic, but has anyone tried feeding explosive precursors to various microorganisms to see if they shit out
anything useful? |
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC110595/
Anaerobic microorganisms (found in common "municipal sludge"  ) can reduce
either 1 or 2 of the nitramine groups in RDX to nitrosamines (one less oxygen atom). Other microbial end degredation products included nitrous oxide,
methanol, formic acid, methane, and carbon dioxide. ) can reduce
either 1 or 2 of the nitramine groups in RDX to nitrosamines (one less oxygen atom). Other microbial end degredation products included nitrous oxide,
methanol, formic acid, methane, and carbon dioxide.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I just had a thought.
Although 1,1,3,3-tetranitro-cyclobutane is not considered melt-castable (there is significant decomposition at its 165 °C melting point),
perhaps 1,1,3-trinitrocyclobutane would be melt-castable.
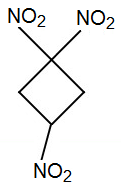
A structurally similar compound, 1,3,3-trinitroazetidine (TNAZ) is melt-castable, having a melting point of 101-103 °C.
Trinitrocyclobutane could potentially be a high-performance replacement for TNT. Just compare molecular formulas,
C4H5(NO2)3
C7H6(NO2)3 for TNT
And unlike the synthesis for TNAZ, preparing cyclobutane derivitives could potentially be much more straightforward.
Partial condensation of formaldehyde with nitromethane under alkaline conditions to obtain 2-nitroethanol (note that 2-nitroethanol is toxic and can
potentially be absorbed through skin contact). 2-nitroethanol then distilled with conc. H3PO4 and NaBr to give 1-nitro-2-bromoethane. This is then
cyclized to 1,3-dinitrocyclobutane in a procedure similiar the one used by Wade (synthesis trans-1.2-Dinitrocyclopropane (DNCP) ).
see the thread in this forum, "1,2-dinitro cyclopropane and nitric acid?" or use the link below,
Quote: Originally posted by Pulverulescent  |
2.6 Synthesis of Compound 6: trans-1.2-Dinitrocyclopropane (DNCP).
Wade and co-workers' procedure<sup>9</sup> for the synthesis of trans-1,2-dinitrocyclopropane
|
An oxidative nitration could then be done to add the third nitro group, 60% yield, using a mixture of sodium nitrite and sodium persulfate in the
presence of potassium ferricyanide*, or in 39% yield with a mixture of sodium nitrite and silver nitrate.
*This procedure is similar to the synthesis of 1,1-dinitroethane from nitroethane, the details of which can be found in this forum.
For comparison, 1,1,3,3-tetranitro-cyclobutane has a density 1.83 g/mL, while TNAZ has a density of 1.84 g/mL. I cannot find any reported values, but
1,1,3,3-tetranitro-cyclobutane is probably slightly more powerful than HMX.
[Edited on 18-1-2012 by AndersHoveland]
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
"Giver of Bad Advice"?
Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Ha-Cough! Hack! Choke!
Giver of emphysema─ by default . . . 
P
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | 1-nitro-2-bromoethane. This is then cyclized to 1,3-dinitrocyclobutane in a procedure similiar the one used by Wade (synthesis
trans-1.2-Dinitrocyclopropane (DNCP) ).
|
I would like to post one potential correction. Reaction of 1-nitro-2-bromoethane with a base may also likely just yield nitroethylene, but this
intermediate should still be able to condense with another alkaline nitroalkane. It might be a better idea not to brominate the whole portion of the
2-nitroethanol all together in the earlier step.
NO2-CH=CH2 + [-]NO2=CH2-CH2-OH --> [-]O2N=CH-CH2-CH(-NO2)-CH2-OH
(There are mentions in the literature of nitroethylene, or 1,1-dinitroethylene, condensing under alkaline conditions with formalin, which is just
another name for formaldehyde. The literature also mentions "condensation of piperylene with nitroethylene". So these types of reactions are fairly
characteristic of nitroethylene.)
[-]O2N=CH-CH2-CH(-NO2)-CH2-OH --> conc. H3PO4, NaBr --> NO2-CH2-CH2-CH(-NO2)-CH2-Br
The latter compound could then be cyclized [into the cyclobutane derivitive] by being made alkaline again with sodium acetate.
I also added a diagram in the other thread to help clarify the chemistry of these types of reactions.
another side note of lesser importance,
Quote: Originally posted by AndersHoveland  |
An oxidative nitration could then be done to add the third nitro group, 60% yield, using a mixture of sodium nitrite and sodium persulfate in the
presence of potassium ferricyanide*.
|
Some other sources in the literature say that this reaction does not work on 1,3-dinitrocylcobutane, but other sources give conflicting information.
The literature clearly shows it to work with azetidine compounds, so there is no reason it should not also work on cyclobutane derivitives.
It is also very important that water not be present if the nitrocyclobutane derivitives are made alkaline. Just as is the case with
nitromethane, the presence of water results in more complex reactions.
also see
"Hydrogenolytic Denitration of Polynitro Compounds",
Raja Duddua, Paritosh R. Davea, Reddy Damavarapub, Rao Surapanenib & Richard Gilardic, pp 2709-2714, Synthetic Communications, Volume 35, Issue
20, 2005
[Edited on 20-1-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Consider this: The bond dissociation energy of carbon monoxide is 1072 kJ/mol, which is higher than even that of diatomic nitrogen N2 at 942 kJ/mol.
In practice, however, carbon and oxygen stored in a molecule are usually already bound with more strength than nitrogen is. A single nitrogen-nitrogen
bond is also much weaker than bonds between other atoms. So theoretically a high nitrogen content can be better. But in practice, taking either
approach to an extreme, either all nitrogen, or all fuel-oxidizer, is likely to result in higher sensitivities.
It may be ideal to have at least one nitrogen (better with 1 or 2 hydrogen atoms on it) act as an electron donating group toward a nitrogenous ring or
nitro groups, adding stability and reducing sensitivity.
5,5′-hydrazinebistetrazole, C2N10H4, does not have any oxygen atoms, and has a detonation velocity of around 7200-7400 m/sec (if I remember
correctly), and has a relatively low sensitivity, (sensitivity to shock under 30 J, friction value under 108 N). HCN forms as one of the main
decomposition products.
[Edited on 17-2-2012 by AndersHoveland]
|
|
|
| Pages:
1
2
3 |
|