Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Searching for vacuum pump
I am looking for vacuum pump with 0.1mmHg vacuum(maxium vacuum)
Anyone know what model is suitable for me and how much is it?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I want to buy JB DV-85N Platinum Series Vacuum Pump
It provide 15 micron vacuum!
I want to use it in acetate pyrolysis reaction(it occur at 250-300c) and in 0.1mmhg reduced pressure
as you knlow acetic acid produced in this reaction and at this pressure there is difficult to liquefied it(acetic acid in .1mmhg boil at <-10 )and
all of my acetic acid will come to my vacuum pump
How can i prevent it?
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Cold trap.Normally dry ice acetone in line before the pump.Have a google plenty on it.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
LN cold trap, big one.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Yep, that'll have to be dry ice or LN. Sucks... {a ha har har}
Unless you go all Garage Chemist on us and build a phase change cooler, but having a decent amount of temperature differential is best. 20C or more.
It may be of interest that when putting acetic acid through Sigma's physical properties calculator, it does not give a result for pressures below
10mmHg, at which point it's boiling at 17.96C. I believe this means it's left the region in which the calculation functions, so it may be worth double
checking what the BP will be in practice.
|
|
|
melvinthedestroyer
Harmless

Posts: 20
Registered: 6-7-2011
Member Is Offline
Mood: 293.15K, 101.325kPa
|
|
Too good to be true?
That's funny, I was JUST about to make a thread regarding the purchase of a vacuum pump.
I am looking for a pump which can evacuate a ~4L apparatus and maintain a minimum vacuum of 10 mmHg.
I figured I would have to drop at least $500 on a pump that could do that, but after a little looking around, I found a website that sold more than
capable pumps for very little. It actually made me wonder if there was a catch, or perhaps something I was not understanding (and thus the thread I
was going to start!)
Here's the site:
http://viot.us/showproduct.php?model=pumps&service=product
According to their specifications, I can get a 0.5mbar (0.375 Torr / mmHg, correct?!) for under $100!
Is there a catch? I thought vacuum pumps were supposed to be expensive!
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
http://www.asknumbers.com/PressureConversion.aspx
| Quote: |
It may be of interest that when putting acetic acid through Sigma's physical properties calculator, it does not give a result for pressures below
10mmHg, at which point it's boiling at 17.96C. I believe this means it's left the region in which the calculation functions, so it may be worth double
checking what the BP will be in practice.
|
I checked the boiling point of acetic acid in this pressure(0.1mmhg) in below site:
http://www.trimen.pl/witek/calculators/wrzenie.html
[Edited on 20-7-2011 by Waffles SS]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
There's ultimate pressure, and then
there's pumping rate. How long will it take you to evacuate 4L with that pump?
P.S. I should have read the specs page before I posted. That $100 pump is rated for "intermittent duty", so there's no way it's going to handle a 4L
evacuation. Also, the way they've rated pumping speed is "Free Air Displacement", which is the speed at atmospheric pressure. The pumping rate will go
down a lot when you get near your ultimate vacuum.
[Edited on 20-7-2011 by watson.fawkes]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
The BOC's and things of this world are overpriced considering what's inside them - I can buy an entire second hand car, complete with combustion
engine, for a similar price.
I have recently heard a warning about the ultra cheap air conditioning pumps and that was from a guy on an AC forum, who was just making his first
phase change cooler (to go on his computer). He left the pump running all day, or overnight I think, to remove all the moisture traces from the system
before gassing it and the motor burnt out on the pump - as per watson picking up on the intermittent thing.
They're all probably made in China. I expect some of the well known brands have parts made in China. But he'd bought it directly from China, so the
return postage was about as much as the pump it's self. Motto being, if buying one of the cheaper ones, get it locally. One could also look at the
motor ratings and compare it to the CFM, to look for one with more solid motor. Alternatively, look at the brands Yellow Jacket and Robinair (Cooltech), as those are used a lot buy the air conditioning people, so they are likely rated for continuous duty. Although, from my
limited experience of air conditioning, I believe it is more normal to vacuum a system out for 15 to 30 minutes, as opposed to overnight.
Pfeiffer says;
| Quote: | | Ultimate pressure is the lowest pressure that is asymptotically approached by the pressure of a blank-flanged vacuum pump under defined basic
conditions without gas inlet. If a pump is operated at ultimate pressure, the usable pumping speed will be zero, as only its own backflow losses will
be displaced. Ultimate pressure is a theoretical value. Today, base pressure is specified instead of ultimate pressure. The conditions for achieving
base pressure are specified in standard ISO 21360-1. As the base pressure must be attained within a specified period of time, it is usually higher
than the ultimate pressure. |
[Edited on 20-7-2011 by peach]
|
|
|
melvinthedestroyer
Harmless

Posts: 20
Registered: 6-7-2011
Member Is Offline
Mood: 293.15K, 101.325kPa
|
|
Thanks for the information!
Considering I am only looking to get to a 10mmHg or better vacuum, I don't ACTUALLY need the pump to perform as the manufacturer states (12 microns
for their better units, but as peach was saying, this doesn't sound like it would even be possible anyway).
If I were to purchase one of the better units (let's say the $277, 7.5CFM unit) from the site, what kind of ultimate vacuum could I ACTUALLY expect to
achieve in a reasonable (30 minutes to an hour) amount of time?
Does anyone have some numbers (ultimate vacuum as described by manufacturer vs ultimate vacuum actually achieved by user) to throw out there? Not just
for these units of course, but for any vacuum pump.
Here's the link to the site again:
http://viot.us/showproduct.php?model=pumps&service=product
Funny enough, I just inherited a pump from a friend of mine.
Here's a site with some specifications:
http://www.centurytool.net/90062_A_Mastercool_Econ_3CFM_1_Stage_Vac_Pump_p/msc90062-a.htm
I have yet to get tubing, pump oil, etc to run a test myself, but does anyone have an opinion on this brand of pump, or a guess as to if it will
achieve 10mmHg or better?
Thanks
[Edited on 20-7-2011 by melvinthedestroyer]
[Edited on 20-7-2011 by melvinthedestroyer]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Theoretically...
To evacuate 4 litres from 760 mmHg to 10 mmHg with a pump flow rate of 3 CFM will take 0.2 seconds.
With 7.5 CFM of flow rate, it'd be 0.0816 seconds.
It depends what you plan to do with the vacuum once it's there. The reason that other guy burnt his out was the amount of time he left it running to
remove traces of moisture. The vacuum will have reached pretty much it's full limit almost instantly.
If you were evacuating it to then do a physics experiment in, you may be able to close the pump off from the chamber and switch it off - provided it's
well sealed.
If you were degassing something (like resin), it'd have to run for a few minutes to get the dissolved gas out before the pressure dropped to the
ultimate.
Running a distillation, those take about an hour to three and all the joints on the glass do tend to leak a bit. If the pressure's not constant, the
temperature will be going wonky and it'll do monster bumps if the pump is reconnected too quickly; so it needs to be left on to keep the boiling
points stable.
10 mmHg is not a particularly harsh vacuum. It's not far off fridge pumps and aspirators. However, a fridge pump has a flow rate of something like 0.1
CFM from memory, so they do take quite a while to evacuate multiple litres.
The base pressure thing is more applicable to industries where they have to continually cycle pumps, in sputtering heads and things that are being
loaded and unloaded at a high rate by a robotic arm for instance. Or in university / laboratory physics experiments, where they have chambers
measuring tens of litres or more and want it down to 0.000000000001 mBar or some other number with a ton of zeros in it; that takes ages, scraping all
the extra zeros on, getting to 1mBar isn't really the hard part.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by melvinthedestroyer  | | Considering I am only looking to get to a 10mmHg or better vacuum, I don't ACTUALLY need the pump to perform as the manufacturer states (12 microns
for their better units, but as peach was saying, this doesn't sound like it would even be possible anyway). |
You're at the point when you'll need to state your application. Initial pump-down isn't the problem, I'd guess, but the maintenance
of the vacuum. (This is why an intermittent duty motor is likely not the right thing for you.) To get a fine point on the question, where's the gas
coming from that you need to keep pumping out? Leaks, desorption, chemical reaction, etc.
|
|
|
melvinthedestroyer
Harmless

Posts: 20
Registered: 6-7-2011
Member Is Offline
Mood: 293.15K, 101.325kPa
|
|
I will be using the pump for distillations. The largest distillation flask that will be used is 2L, and the largest receiving flask will be 1L. To err
on the side of caution, and to take into account any other devices I might be affixing to the apparatus, I planned on evacuating 4L of air. I might
actually be purchasing a 3L flask soon, so I may as well assume 5-6L just in case.
All joints are 24/40 and will be sealed with silicone grease, of course.
So I would need to run the pump continuously throughout the distillation? I figured, even with leaks, I could run the pump, monitor the pressure, if
it got a little too high, turn the pump on, repeat... based on what peach and watson are saying, however, this sound like it would be problematic as a
result of bumping. Am I understanding this correctly?
So I would need a continuous duty vacuum pump? Returning to the first link I provided, would the $277, 7.5CFM model be acceptable then? Actually, according to this seller, there are several other cheaper models
that are acceptable for continuous duty, although they do not mention lab use in the comments section...
Any idea if the matercool pump I mentioned would be acceptable? I swear I'll just test it for myself soon!
Thanks for the great information and quick replies!
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
For distillations, you will want the pump running continuously. It can be hard enough to get stable temperatures when things are close to each other
without the pressure waving up and down. Boiling can also be erratic under vacuum, even when it's constant.
A 1 CFM pump would do for at home chemistry (in fact, I have both a 7.5 and a 1.3 or something sized pump, the 1.3 works fine, the air is gone almost
as soon as it's connected). In a distillation where the goal is to get something as pure as possible, that means turning the heat right down so it's
coming over slowly. The vast majority of your time will be spent waiting for the material to come over, as opposed to waiting for the volume to vacuum
down. When a distillation is running correctly, i.e. the vapour is fully condensing and staying condensed, the flow rate through the pump will be 0,
because nothing will actually be leaving.
The Mastercool pump will easily evacuate the glass, quickly and to the pressure you want. The only question is, will it be okay to leave it running
for 3 hours constantly. To be on the safe side, go with 6 hours. If you have a manual for it, flick through it and see if it mentions duty cycles
anywhere in there, or switching it off after a set period of time. Even stuff from China often includes that detail.
If not, try phoning Mastercool and ask.
If that Mastercool can put up with being left on, I'd say save your cash and spend it on something else. If your friend has been using the pump on a
regular basis to suck water and refrigerants out of AC system, you might want to instead give it an early Christmas and buy it a new bottle of oil.
|
|
|
melvinthedestroyer
Harmless

Posts: 20
Registered: 6-7-2011
Member Is Offline
Mood: 293.15K, 101.325kPa
|
|
Thank you for the great information, peach!
I will certainly give it some fresh oil! Chances are, it won't need it though; my friend used it maybe once or twice before giving it to me. It has
spent the majority of its life sitting on a shelf! It will be getting its "Christmas present" anyway.
Again, thank you! You have no idea how much I appreciate the information and quick replies. I just hope the creator of this thread is having as much
luck.
I will try to remember to post an update as to the success/failure of the pump once I get everything I need to run a test.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Glad it's sorted.
If you're going into vacuum distillations, you'll want to print a copy of this (nomograph) and either cover it in sticky back plastic or laminate it.
Without doubt the handiest thing around for vacuums; that's free anyway.
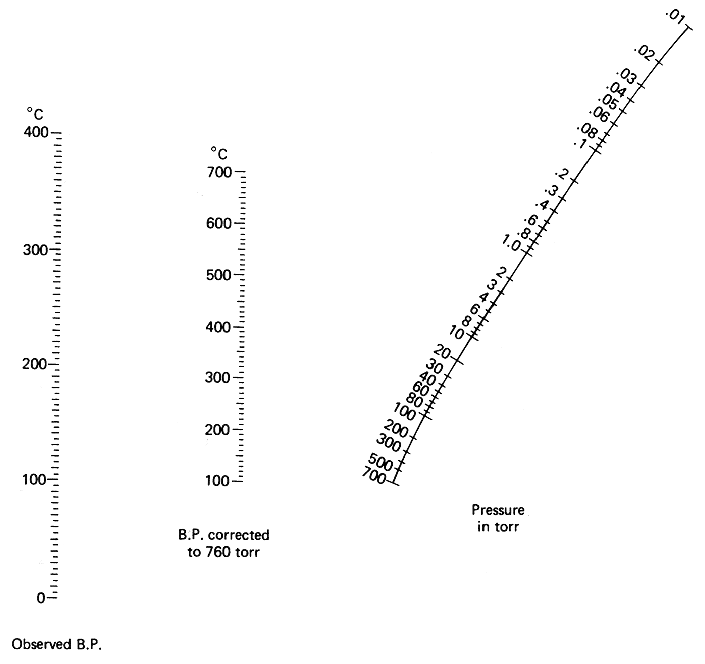
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
How much pressure one standard rb flask can take?can it take 0.1mmhg?(or it may break)
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
What's ΔP
in this case? It's about -1 atm. Variations in barometric pressure completely swamp the difference between 0.1 torr and 10 torr. Bench-sized glass in
good condition will handle that 1 atm. "Good condition" is an important criterion: no cracks, consistent wall thickness, etc. If you add other forces
to the glass, say, tapping it with a metal spatula, you can trigger implosion. The reason people use heavy-wall ware is to provide a safety margin.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by watson.fawkes  | | What's ΔP in this case? It's about -1 atm. Variations in barometric pressure completely swamp the difference between 0.1 torr and 10 torr.
|
What is -1 atm?-760 mmhg?!I think ultimate vacuum is 0 mmhg
What mean ΔP?temp? i will heat my rb flask to 300c
What happen if water vapor come into my vacuum pump?I decide to dry my sample and i want to connect my desicator directly to my pump,sure water
vapor(from my sample) will come into pump
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Watson has it dead on.
By Delta P, he means the pressure differential from one side of the glass to the other. Since the atmosphere doesn't change much, removing all the gas
inside the flask means it'd be -1 atm (minus the atmosphere). He's used the minus sign to show the pressure differential has come about by removing
gas from the inside, making the assumption (correctly) that you mean you'll be sucking the air out, as you only stated 'pressure' and pressurising
glass is another thing.
1 atmosphere is about 1000mBar. If I look at my barometer, it usually only moves from 980 to 1020. So the pressure on the outside of the glass only
changes by about 2%.
The vacuum Melvin is talking about is 10mbar, which means his pump is changing the pressure inside by 99%. The pump is dictating the vast majority of
the pressure differential on the glass walls. Going to 1mbar changes it to 99.9%, so barely any practical change in terms of the force on the glass
once 90 -> 99% of the air is already gone.
Jointed glassware, like an RBF, can withstand a complete vacuum; I mean, every single molecule gone inside. Going from 90% -> 99% (vacuums) to
99.99999999999999999% (outer space) means nothing in terms of the forces on the walls. It's like building a house and then balancing a pea on top of
it. That extra pea isn't going to mean much in terms of the pressure on the foundations.
Provided the glass is chip and scratch free, it'll be fine. And, as he says, provided you don't knock it around whilst it's under vacuum or expose it
to some crazy rapid temperature change at a specific point on the surface. If the whole of the glass is at a uniform normal temperature (I wouldn't go
far over 300C), it'll also be fine. You'd have to be distilling something fairly special to need it at 300C under vacuum, as most things of interest
tend to start boiling a lot closer to 100 under vacuum.
Baffled glassware is usually not suitable for use under vacuum, because the baffles create ideal spots for stress to concentrate on the walls, making
it likely it'll implode. But I don't think I've seen a single person on here actually using baffled glass.
In terms of drying things under vacuum, you should either use a trap that can chemically absorb the moisture before it reaches the pump or use a dry
ice / liquid nitrogen setup. It depends how expensive your pump was, if it has a ballast port on it and how much water you're thinking of removing.
For instance, AC guys use pumps specifically to remove moisture, but there is barely any in there, it's not like they're connecting the pump to a big
soggy pile of organic matter. It's a good idea to remove as much of the excess moisture at atmospheric pressure prior to using vacuums on it, so's to
limit the amount of trouble the water is going to cause.

|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Thanks Dear Peach,
Now i am trying two different reaction:
-Acetate pyrolysis under vacuum
-Produce Hydroxylamine Hydrochloride from Hydroxylammonium Sulfate(by Barium Chloride)
In the first route my temp will be 300c and pressure at .1(torr).
And in the second route after reaction between Hydroxylammonium Sulfate and Barium Chloride,Hydroxylammonium Chloride will produce and this is not
possible to boil solution to get Hydroxylammonium Chloride because at ~150c it will decompose(Nitrous oxide will form),Then i should boil solution
under vacuum and then dry wet hydroxylammonium chloride in desicator (under vacuum)
I think i should use vacuum Erlenmeyer(Büchner flask) for first route(it seems it is more stable at 0.1 torr reduced pressure.)
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  | Glad it's sorted.
If you're going into vacuum distillations, you'll want to print a copy of this (nomograph) and either cover it in sticky back plastic or laminate it.
Without doubt the handiest thing around for vacuums; that's free anyway. |
I have several copies of this I had printed on cardstock, with tables of common solvents and properties on the back, then laminated with the heaviest
laminate available. I second your recommendation!
|
|
|
melvinthedestroyer
Harmless

Posts: 20
Registered: 6-7-2011
Member Is Offline
Mood: 293.15K, 101.325kPa
|
|
I didn't have the time to perform a proper test, but the Mastercool 3CFM, 1 stage pump was capable of bringing water to a heavy boil at room
temperature nearly instantly. I was using a 500mL RBF and about 150mL of tap water.
I then tried again with water at about 0C. The water looked as if it had become saturated and began a very, very mild bubbling, but did not really
boil. I will conduct a test with a (working) thermometer soon, as it appears that the one I was using isn't actually working properly. I will then get
a reasonable estimate of the actual ultimate vacuum.
My guess is it is somewhere between 4mmHg and 9mmHg. Just what I was looking for!
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Excellent. Keep in mind that it won't give it's lowest pressure reading if the water vapour is going out to the pump, as the pump is then shifting
something and the ultimate pressure is defined at zero flow rate; e.g. the thing under vacuum is fully condensed. They have almost zero flow rate as
they approach that value, so it doesn't take much to shift it around (this is why UHV chambers need to be spotlessly clean and all the materials need
to be specified not to contribute any vapour pressure to the chamber; e.g. iron based metals, not aluminium).
Your bottom pressure is probably a bit lower than the one calculated if the pump was straight on the flask. Distilling something with a high boiling
point is likely to show that up.
You could either do that now to check the value or just rest assured that it will be slightly lower. Pick something with an atmospheric BP around
250->300C.
[Edited on 29-7-2011 by peach]
|
|
|