| Pages:
1
2 |
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
Mercury vapor under UV light...
<iframe sandbox width="560" height="345" src="http://www.youtube.com/embed/JABbofwD3MI" frameborder="0" allowfullscreen></iframe>
not my video...
but this is pretty scary.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
That explains the glow I get when I turn on my UV Bug trap
after spilling 6 kg on Hg on the rug! The vacuum cleaner 'bout
choked trying to clean it up.
The ever useful Mellor's Inorganic and Theoretical Chemistry
(Vol. IV, pg. 722) says:
F. Glaser [Zeit. Elektrochem., 9. 11, 1903] measured the loss
which occurs with electrolytic mercury per sq. cm. of surface
area per hour, and found 0.002 mgrm. was lost at room
temp......
The saturation point for mercury in air at 20oC is 15mg/m3.
Years back I got a nice UV glow while adding wet Cl2 (from HCl
and KMNO4) to Hg vapour. (Boiling Hg.)
[Edited on 20-8-2011 by The WiZard is In]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by The WiZard is In  |
That explains the glow I get when I turn on my UV Bug trap
after spilling 6 kg on Hg on the rug! The vacuum cleaner 'bout
choked trying to clean it up.
The ever useful Mellor's Inorganic and Theoretical Chemistry
(Vol. IV, pg. 722) says:
F. Glaser [Zeit. Elektrochem., 9. 11, 1903] measured the loss
which occurs with electrolytic mercury per sq. cm. of surface
area per hour, and found 0.002 mgrm. was lost at room
temp......
The saturation point for mercury in air at 20oC is 15mg/m3.
Years back I got a nice UV glow while adding wet Cl2 (from HCl
and KMNO4) to Hg vapour. (Boiling Hg.)
[Edited on 20-8-2011 by The WiZard is In] |
Excuse me? You did what? You vacuum cleaned 6 kg of mercury? Have you completely lost your mind?
Mercury is NEVER, EVER vacuum cleaned because that creates a heavy contamination of the area due to very fine spheres of the metal being thrown
around, most of them which are almost invisible.
Oh my god, I can't believe it. I simply can't believe how can someone be so irresponsible.
And what's about this glow you're talking about? Mercury vapor doesn't glow when exposed to UV light. It is impossible to see the vapor. You can see
the shadow only, if you make the right experiment setup. Like I did here.
Dude, are you serious? Are you serious with what you're talking?
Do you know the consequences of your actions?
Oh my god, I simply can not believe the shit I'm reading on this site. 
The things you wrote in this post clearly show that you fail as an amateur chemist and that you either need to learn the basics, or leave it all
alone.
[Edited on 21-8-2011 by Endimion17]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  |
Excuse me? You did what? You vacuum cleaned 6 kg of mercury? Have you completely lost your mind?
Mercury is NEVER, EVER vacuum cleaned because that creates a heavy contamination of the area due to very fine spheres of the metal being thrown
around, most of them which are almost invisible. |
My hero.
http://www.nejm.org/doi/full/10.1056/NEJM200006153422405
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
very interesting wizard.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  |
Excuse me? You did what? You vacuum cleaned 6 kg of mercury? Have you completely lost your mind? Mercury is NEVER, EVER vacuum
cleaned |
...should we tell him?
Anyway, thanks for sharing I didn't know the vapor cloud was visible that way, very interesting.
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
That video is pretty scary. Playing with mercury doesn't seem dangerous at all. But actually seeing this toxic vapor really makes one have
the realization that mercury is a dangerous substance.
[Edited on 21-8-2011 by redox]
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|

___
Loved the video. I have always been scared of mercury and have yet to play with it. This re-affirmed my fears I guess.
Cumulative...
[Edited on 21-8-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
In the video is the dish being heated from below ?
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
| Quote: |
Ohm man today sucked.I broke a mercury thermometer.I was cleaning the wall of my lab with some ammonia really nice black smudge coming of.There was a
thermometer hanging on the wall, it was an old one and the mercury had split because apparently it was lying down for a long time.Anyway somehow I and
my stupid head tipped the thing of the hook and it fell down on my desk.It god damn shattered.   So yeah i had tiny drops of Hg all over the fucking table top.I
spent 2 hours of cleaning it with an eye dropper and still not convinced that it is all gone.I used the last of my sulphur on the floor.Now ill be
putting the radiator at max with the window open hoping to evap all of it.Maybe ill put my gassmask on and use a blow-dryer.What a way to start your
freaking day.Any one got some tips? So yeah i had tiny drops of Hg all over the fucking table top.I
spent 2 hours of cleaning it with an eye dropper and still not convinced that it is all gone.I used the last of my sulphur on the floor.Now ill be
putting the radiator at max with the window open hoping to evap all of it.Maybe ill put my gassmask on and use a blow-dryer.What a way to start your
freaking day.Any one got some tips? |
| Quote: |
Well i cleaned up the mess.Had an electric heater running all night with the window open.This morning i vacuum cleaned my whole lab.Had a ventilator
running and I was wearing a gas mask.Maybe iam over reacting but i hate the idea of chronic poisoning.I feel quite convinced that its fine now, my lab
has never been this clean.Also I have a nice sealed of glass tube with some Hg. |
I'm quoting myself here,
Oke so I might be paranoid, but spilling 6kg and vacuuming it omfg.
Your joking right?
[Edited on 21-8-2011 by User]
What a fine day for chemistry this is.
|
|
|
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
he said its close to room temp... he only heated it with his hands a bit while holding the dish.
i read it in comments bellow on youtube..
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  |
Excuse me? You did what? You vacuum cleaned 6 kg of mercury? Have you completely lost your mind?
And what's about this glow you're talking about? Mercury vapor doesn't glow when exposed to UV light. |
Sure it does when I use my table top Synchrotron light source
for my Bug Light Trap, granted the soft X-Ray it emits may
help.
On the positive side I was able to sell the children for medical
experiments and buy another house with the money. I probable
should have told the buyer of the spill... but heck mercury is
thicker than water and damn near most any thing liquid at
room temp.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I have no idea what's going on around here, but if some of you aren't joking, your health is in serious danger. It might not be apparent now, but as
you're getting older and older, you'll start to experience unpleasant symptoms.
Getting old with chronic mercury poisoning is not something I'd like to experience.
In case no one saw the link, I'm embedding this here:
<iframe sandbox width="480" height="390" src="http://www.youtube.com/embed/Pgy_Nky8wMg" frameborder="0" allowfullscreen></iframe>
I was planning to simulate a spill in a tray, but I wasn't exactly in the mood of cleaning the mess afterwards.
In any case, the video shows that water is capable of greatly reducing the fuming.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: | [rquote] Ohm man today sucked.I broke a mercury thermometer.I was cleaning the wall of my lab with some ammonia really nice black smudge coming
of.There was a thermometer hanging on the wall, it was an old one and the mercury had split because apparently it was lying down for a long
time.Anyway somehow I and my stupid head tipped the thing of the hook and it fell down on my desk.It god damn shattered.   So yeah i had
tiny drops of Hg all over the fucking table top.I spent 2 hours of cleaning it with an eye dropper and still not convinced that it is all gone.I used
the last of my sulphur on the floor.Now ill be putting the radiator at max with the window open hoping to evap all of it.Maybe ill put my gassmask on
and use a blow-dryer.What a way to start your freaking day.Any one got some tips? So yeah i had
tiny drops of Hg all over the fucking table top.I spent 2 hours of cleaning it with an eye dropper and still not convinced that it is all gone.I used
the last of my sulphur on the floor.Now ill be putting the radiator at max with the window open hoping to evap all of it.Maybe ill put my gassmask on
and use a blow-dryer.What a way to start your freaking day.Any one got some tips? |
Sorry sulphur is an urban legend.
Management and control of Hg exposure
American Laboratory July, 1988
David N Easton
Scanned! And you know what that means!!
The following paragraphs are moved to the front. [djh]
-------------------------------
Mercury spill cleanup procedures:
Because mercury will disperse into fine droplets throughout the area where it is
spilled, an effective cleanup procedure requires two steps. Always wear rubber
gloves and take care to avoid spreading the spill through inadvertent contact.
Wear appropriate respiratory protection if testing shows that levels of airborne
mercury are high.
1) A trap consisting of a filter flask connected to a vacuum source at the
side-arm and a length of Tygon tubing at the inlet is used to collect all visible
droplets. A Pasteur pipet at the inlet end of the tube facilitates the pickup. A good
flashlight is essential for finding fugitive droplets.
2) After the gross contamination has been removed, sprinkle the entire area of
the spill with a liberal application of elemental zinc powder. Dampen the zinc
powder with dilute (5-10%) sulfuric acid solution to create a paste-like
consistency. Work the paste into the contaminated surface with a sponge or a
brush. After the paste dries to a light gray color, it may be swept up for routine
disposal. The residual material is removed with soap and water.
This procedure results in an amalgam of mercury and zinc (the acidic solution
shifts the equilibrium toward the bound product). The more conventional
treatment with calcium polysulfide or flowers of sulfur merely reduces the vapor
pressure by coating the droplets. Subsequent frictional forces can disrupt this
coating and result in additional vapor release.
------------
WHEN A 5-lb bottle of elemental mercury was dropped by an employee of the
University of Virginia's Hospital Supply Storeroom, broken glass and larger
mercury pools were hastily swept up. The Office of Environmental Health and
Safety was called to pick up the waste and survey the damage.
The severity of the problem was realized as soon as the cleanup crew arrived.
In just 30 min, storeroom personnel had tracked mercury throughout the area.
Fugitive mercury droplets had rolled everywhere.
The storeroom was immediately shut down and the State Department of
Emergency Services was called in, as a spill of this magnitude exceeded the
hospital's cleanup capabilities.
The problem with any mercury spill is that mercury easily vaporizes at room
temperature, where it can be breathed or absorbed through the skin. Prolonged
exposure to mercury vapor adversely affects the nervous system. Symptoms
may include irritability, depression, vivid dreams, inflammation of the gums,
insomnia, loss of memory, and/or concentration and constricted visual fields.
The OSHA (Occupational Safety and Health Administration) permissible
exposure limit (PEL) for Hg is 0. 10 mg/m 3 and the ACGIH threshold limit value
(TLV) for Hg is 0.05 mg/m 3 . Both of these standards are based on an exposure
for an 8-hr day.
It takes very little mercury to create an unsafe environment. Quantities as low
as I mL can evaporate over a period of time and contaminate millions of cubic
feet of air to levels in excess of allowable limits.
The airborne concentration of mercury vapor was measured in the storeroom,
and found to be above 0.3 mg/m3. Improper spill cleanup techniques had broken
the droplets into smaller particles that collected in porous surfaces and floor
cracks. Mercury had been tracked all over the room on shoes and the wheels of
medical carts.
The following day, the Department of Emergency Services helped clean up the
remaining mercury with a special mercury vacuum cleaner. Results were tested
with a direct-reading mercury analyzer. Testing showed that levels were then
below 0. 1 mg/m 3 . The Department left the analyzer with the hospital for con-
tinued testing.
By the following morning, mercury vapor levels had dropped to 0.05--0.06
Mg/M3. Pans of the storeroom were reopened for essential supplies and orders.
Unfortunately, by afternoon, the analyzer
showed that mercury vapor levels were starting to rise again. 'Me area was
cleaned using a paste of zinc powder, a technique that has become an integral
part of the cleanup procedure (Figure 1).
By that time, 22 employees were identified as having been potentially exposed to
excessive mercury levels. They received a medical evaluation consisting of
medical history, physical exam, laboratory tests, and computerized neurobe-
havioral testing. Laboratory tests included spot urine tests, 14-hr urine
follow-ups, and blood mercury determinations. All the test results were negative.
While the state's mercury detector was still on loan, some adjacent hospital
areas were surveyed for excessive mercury vapor concentrations.
The first location was the medical repair room where employees of the Clinical
Engineering Department repair such mercury-filled instrumentation as incubator
thermometers, sphygmomanometers, and other differential pressure devices.
(Wall mounted units are frequently knocked over in patient rooms when beds are
adjusted; and tripod units are overturned on a regular basis. The mercury-filled
tubes shatter upon the slightest impact,)
Equipment in need of repair or recalibration is sent to the Medical Repair
Room. Although some mercury from broken equipment can contaminate patient
rooms, most of it is usually found on the repair room floor and work counters.
Employees have reported that they scooped up larger mercury puddles and
threw them into open trash cans and vacuumed up the rest with a regular
household vacuum cleaner. They also stomped on tiny droplets until they were
no longer visible.
The mercury detector confirmed the suspected high levels in the repair room.
Air samples in the room showed average mercury vapor levels of 0.3 mg/m 3 ;
an analysis of the vacuum cleaner's interior levels pushed the instrument's
gauge off scale.
Twenty-eight employees of the Clinical Engineering Department were identified
as either chronically or potentially exposed to elevated mercury levels, including
those who repaired manometers, those who worked in the contaminated repair
room, and others who worked in adjacent offices.
After testing, none of the employees were found to exhibit signs of mercury
poisoning, although four employees were considered to have elevated blood
mercury levels. Four others claimed eyelid fasciculations and depression. Two
admitted increased irritability and insomnia; two others excitability, forgetfulness,
anorexia, and occasional nausea.
It was obvious from the testing that mercury-contaminated areas needed to be
cleaned immediately and kept mercury-free. Employees obviously needed
education about the dangers of mercury vapor and instruction on proper cleanup
procedures.
First, steps were taken to control access to all mercury-based materials by
running all mercury acquisitions through the University Office of Environmental
Health and Safety. The mercury is now stored there and not in satellite facilities
like the supply storeroom.
Next, a portable mercury vapor analyzer was purchased so that mercury spills
could immediately be analyzed for elevated mercury vapor levels. The analyzer
selected (Figure 2) provides fast, accurate, and specific mercury-only readings
through the use of gold film technology. Instruments that respond to
interference’s such as organic materials are confusing to read when the mercury
content of cart wheels or shoes is being analyzed.
Complete mercury spill cleanup kits were assembled. A spill response
procedure was published that defined everyone's responsibilities.
Most importantly, a large employee population was made aware of the
potential hazards of mercury (Figure 3). Mercury Is highly toxic-however benign
or fascinating it looks. The special handling of mercury necessary for mercury
cleanup was stressed because of the compound's easy vaporization and fast
absorption into porous surfaces and materials.
The new procedures caused the phones in the Office of Environmental Health
and Safety to ring almost immediately. It became evident that mercury was
being spilled round the clock.
A skeleton mercury cleanup crew was put on duty at all hours. The hospital
housekeeping staff was selected as the surrogate spill-response team.
Although a few problems were encountered initially communicating safe
cleanup procedures, the staff soon took great pride in their new role. Frequent
training and hands-on experience is currently provided for new personnel. With
this assistance, we know when, where, and by whom mercury is spilled. It has
also been very effective to devote a few minutes of Right-to-Know lectures to
inform all
university employees about the use and abuse of mercury.
The mercury vapor analyzer is used routinely to test patient rooms, repair
areas, dental clinics, and neonatal incubators, and to test deletion after mercury
spills. Problems are still encountered. In the first survey of 24 incubators, one
showed a low level mercury vapor contamination (0.003 Mg/M3) ; another signif-
icant levels (0.020 mg/m 3). Fortunately the badly contaminated unit was used
exclusively as a transport unit and any particular infant's exposure was brief. The
units had to be completely dismantled and decontaminated.
The procedures that were developed for mercury spill cleanup follow. If you
spill mercury (any amount):
1) While wearing rubber or plastic gloves, carefully pick up any broken glass
and debris, Take care not to spread the mercury. Dispose of the glass in a
zip-lock plastic bag or other proper disposal container.
2) Consolidate the spill as much as possible by using a thin piece of cardboard
or plastic. Cordon off the area to avoid traffic and dispersal of the material.
3) Notify your supervisor and call the University Housekeeping Office. A
representative will respond to pick up spill wastes for proper disposal and to
clean up the rest of the spill with proper equipment. Mercury does not evaporate
rapidly, so a delay of an hour or two should not result in the accumulation of
hazardous concentrations; however, the spill must be cleaned up during the work
shift in which it occurred unless specific notification is provided to the supervisor
of the next shift.
4) Call the Environmental Health and Safety Office as soon as the spill is
cleaned up or if there is a delay of more than two hours from the time the spill
was reported. During regular work hours, a representative of the Environmental
Health and Safety Office will respond to monitor the area where the spill has
been cleaned.
If you call to report a spill and cleanup after regular working hours, leave a
message according to the recorded instructions. The recording device will list
home telephone numbers of our staff members in the case of a significant
emergency.
Mr. Easton is a Certified Industrial Hygienist at the Universirty of Virginia,
Environmental Health and Safety Office. Charlottesville, Virginia. This paper was
excerpted from a lecture by the author at the American Industrial Hygiene
Conference in Montreal, Canada, June 1987.
----------
The MSDS for my Baker Mercury Spill kit claims the following ingredients:—
Cinnabsorb®
http://www.jtbaker.com/msds/z6057.htm
Zinc 90.8 - 92.4%
Zinc oxide 0 - 1.4%
Lead 0 – 0.2
Sulfamic Acid 7.6%
Resisorb®
http://www.jtbaker.com/msds/Z6140.htm
Iodine 5 - 25%
Activated Carbon 75 - 95%
[Edited on 21-8-2011 by The WiZard is In]
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Thanks, Jor pointed this out in the original thread (bad days in the lab).
Zinc seems an effective way to clean it up, better yet dont spill it 
What a fine day for chemistry this is.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Wizard:
No more jokes for you until you master the use of emoticons! 
(See? That wasn't too hard, now was it!  ) )
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
gotta admit the knee-jerk reactions never get old. in the current state of chemophobia, in a few years, hazmat teams will show up to evaluate and
sanitize baking soda volcano experiments. yeah yeah i know that mercury and many other chemicals and elements are hazardous, but some of these cats
today have no idea what people have lived through in the past.
now let the flaming begin.....
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
Man sitting in/on a tank of mercury 

|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by Rogeryermaw  | gotta admit the knee-jerk reactions never get old. in the current state of chemophobia, in a few years, hazmat teams will show up to evaluate and
sanitize baking soda volcano experiments. yeah yeah i know that mercury and many other chemicals and elements are hazardous, but some of these cats
today have no idea what people have lived through in the past.
now let the flaming begin..... |
The problem with elemental mercury isn't in acute toxicity. It's in its horrible property to make extremely small spheres upon disturbing. They really
get everywhere. I once had a small accident near my cellphone and few days later I looked at the buttons and other details with a low powered
microscope. Guess what I found between dead skin cells and hair. Few extremely small drops. I don't panic because of it. 2-3 drops the size of ant's
dick won't make a matter, but it gives a nice chilling feeling to know just how serious any spill is.
The hazmat teams are out there not because the area is all of a sudden very dangerous, but because the area needs a thorough cleaning in order to stop
fuming. And when I say thorough cleaning, I mean chernobyl style.
Regular people don't have the knowledge how to clean that mess.
And it just shows how small atoms are and how many of them are in even minute quantities of matter.
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
A thick dusting of sulfur and then some heat (ambient, just a very warm room), followed by a good vacuuming (vented outside) is necessary for any
spill.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17now let the flaming begin  |
The hazmat teams are out there not because the area is all of a sudden very dangerous, but because the area needs a thorough cleaning in order to stop
fuming. And when I say thorough cleaning, I mean chernobyl style.
Regular people don't have the knowledge how to clean that mess.
And it just shows how small atoms are and how many of them are in even minute quantities of matter. |
MMWR. 1991,40:393 395
[Mortality and Morbidity Weekly Reports, from 1993 are available on the Web.]
Acute, Chronic Poisoning, Residential Exposures to Elemental Mercury — Michigan, 1989 1990
FROM MAY 1989 through November 1990, eight episodes of elemental mer¬cury exposure in private residences or schools in the United States were
re¬ported to the Agency for Toxic Sub¬stances and Disease Registry (ATSDR). The case studies in this re¬port document two of these episodes (both
in Michigan) of residential mer¬cury poisoning one involving acute mercury exposure, and the other, chronic exposure to elemental mercury. These
episodes illustrate the differing clinical and toxicologic manifestations of acute and chronic mercury poisoning. Episode 1. On August 7, 1989, four
adult occupants (two men and two women ranging in age from 40 to 88 years) of a private home were hospitalized for eval¬uation of nausea, diarrhea,
shortness of breath, and nonspecific chest pain. Dur¬ing hospitalization, the patients experi¬enced progressive dyspnea and pulmo¬nary
insufficiency. On August 11, investigators learned that one of the pa¬tients had been smelting dental amal¬gam in a casting furnace in the basement
of the home in an attempt to recover sil¬ver from the amalgam. Mercury fumes released during the operation appar¬ently had entered air ducts in the
base¬ment and had circulated throughout the house.
Because of this mercury vapor expo¬sure, chelation therapy with dimerea¬prol was initiated in the patients. On Au¬gust 12, urine mercury
concentrations from three of the patients ranged from 94 to 423 ug/L; serum mercury concen¬trations from two patients were 127 and 161 ug/L.
Despite chelation therapy and vigor¬ous ventilatory support treatment, the condition of the patients continued to deteriorate. All of the patients
died within 11 24 days after exposure to the mercury vapor. The cause of death was considered to be mercury poisoning, which resulted in adult
respiratory dis¬tress syndrome and subsequent respi-ratory failure. Postmortem mercury concentrations in organs from the four patients were 300 2100
ug/g (kidney), 3 2400 ug/g (liver), <1 100 ug/g (brain), and 1 150 ug/g (lung); concentrations in blood ranged from 58 ug/L to 369 ug/L.
Measurements of ambient indoor air concentrations of mercury taken 11 18 days after the exposure were as high as 786 ug/m3 in the basement and 912
ug/m3 on the first floor. The house was extensively cleaned to reduce the mer¬cury contamination; however, decon¬tamination efforts did not reduce
indoor air mercury concentrations to an accept¬able level, and the house was subse¬quently demolished.
Episode 2. On August 21, 1989, a young girl was admitted to the hospital be¬cause of impaired gait. She was diag¬nosed as having a postinfectious
viral syndrome and was discharged on Au¬gust 23. On September 11, she was re¬admitted to the hospital when she could no longer walk. On September
19, an older sister of the patient was admitted to the hospital with similar symptoms. Clinical evaluation of both girls revealed numbness in the
fingers and toes, ab¬sence of deep tendon reflexes, elevated blood pressure, and an elevated level of protein in the cerebrospinal fluid Mer¬cury
poisoning was diagnosed, and che¬lation therapy was started in the two children. Subsequently, on October 3, their asymptomatic brother was
hospitalized for a chelation challenge, which detected a substantial mercury load.
After chelation therapy, the brother remained asymptomatic, and the older sister improved and was discharged from rehabilitation therapy. The index
patient had numerous residual neurologic abnor¬malities, including visual field defects, mild upper and lower extremity weak¬ness, and some
emotional lability.
Subsequent investigations revealed that earlier that summer about 20 cm3 of liquid mercury had been spilled in the boy's bedroom. Examination of the
house using a mercury vapor analyzer detected indoor air mercury concentrations of 1040 ug/m3. Clean up efforts included re¬moving carpet from
several areas in the house, replacing the carpet and wooden subfloor in the bedroom and commercially cleaning all other carpet and furniture.
CDC Editorial Note: Although the toxic properties of elemental mercury have been recognized since at least the 1500s, occupational and residential
ex¬posures to mercury remain a source of poisoning.
Although death is an infrequent out¬come of acute exposure to mercury, the first episode described in this report il¬lustrates the clinical
progression follow¬ing exposure. Patients are usually asymptomatic during the first 14 hours following acute exposure to high air con¬centrations of
mercury vapor. Symp¬toms start abruptly and may include fe-ver, chills, nausea, general malaise, and respiratory difficulties (shortness of breath,
pain and tightness in the chest, and paroxysmal coughing). In severe cases, pulmonary edema may cause death within a few days. (1,2)
After inhalation, elemental mercury is readily absorbed through the alveolar membranes and transported by blood to the brain and other tissues of the
nervous system. Mercury is rapidly converted by the blood to mercuric ions, which are then excreted in the urine and feces. Diagnosis of mercury
toxicity is aided by the detec¬tion of elevated concentrations of mer¬cury in blood or urine samples. Back¬ground urine concentrations of mercury
in persons with no unusual exposure to mercury range from 1 to 25 ug/L; 95% of such urine samples contain <20 ug/L. (1) Although urine mercury
concentrations correlate poorly with manifestations of mercury poisoning, symptoms may ap¬pear when the urine mercury concentra¬tions exceed 300
ug/L. (3) In unexposed persons, blood mercury concentrations are usually <3 ug/L, but may be substantially higher in persons with a high dietary
intake of fish.
Residential and occupational cases of mercury poisoning more commonly re¬sult from chronic exposures, as illus¬trated by the second episode
described in this report. Spilled mercury gravi¬tates to cracks in the floor and into the pile of carpets. Even though it may not be visible, the
mercury can slowly vol¬atilize indoors and may lead to chronic mercury poisoning through inhalation exposure. Vacuuming a contaminated area may
facilitate the spread of mer¬cury vapor throughout the house.
The potential for indoor mercury expo¬sure is increased when indoor air ex¬change is reduced (e.g., when doors and windows are kept closed). Warm
air from heating ducts and vents may enhance vol¬atilization when circulated over spilled mercury. Mercury vapor concentration is likely to be higher
near the floor, and chil¬dren may be exposed to higher concen¬trations of mercury than adults.
The vagueness of the early clinical signs of central nervous system (CNS) toxicity characteristic of mercury poisoning often result in misdiagnosis.
If exposure to mercury continues, the severity of symp¬toms may progress as a function of mer¬cury concentration, length of exposure, and individual
sensitivity. The CNS tox¬icity of mercury is both neurologic and psychologic. Fine tremors in the fingers, eyelids, and lips are early signs of
mer¬cury toxicity. Tremors in the hands and arms may interfere with precision move¬ments and impair skills such as handwrit¬ing. Common
psychopathologic symp¬toms include depression, irritability, exaggerated response to stimuli, exces¬sive shyness, insomnia, and emotional
in¬stability. (1,2)
Potential sources of elemental mercury in the home include mercury switches and mercury containing devices such as ther¬mostats, thermometers, and
barometers. Family members may also bring into the home elemental mercury obtained from laboratories, dental offices, or other in¬dustrial sources.
In the ATSDR Toxic Substances Profile for Mercury, the minimal risk level (MRL) for chronic inhalation exposure to elemental mercury was determined to
be 0.3 ug/m3. (1) An MRL is an estimate of the daily human exposure to a chemical that is likely to be without an appreciable risk of deleterious
(noncarcinogenic) effects during a specified period of exposure. Chronic inhalation exposure to elemental mercury concentrations below the MRL would
not be expected to result in adverse health effects. (1)
References
1. Agency for Toxic Substances and Disease Reg¬istry. Toxicological profile for mercury. Atlanta: US Department of Health and Human Services, Public
Health Service, Agency for Toxic Sub¬stances and Disease Registry, 1989; publication no. ATSDR/TP 89/16.
2. Gerstner HB, Huff JE. Clinical toxicology of mercury. J Toxicol Environ Health 1977;2:491 526.
3. Knight AL. Mercury and its compounds. In: Zenz C, ed. Occupational medicine: principles and practices. 2nd ed. Chicago: Year Book Medical
Pub¬lishers, 1988:590 6.
4. Occupational Safety and Health Administration. Air contaminants: final rule. Federal Register 1989;54:2415 6.
Reported by: C Taueg, MPH, Wayne County Health Dept; DJ Sanflhppo, MD, Grand Rapids; B Rowens, MD, Detroit; J Szejda, Ottawa County Human Svcs,
Holland; JL Hesse, MS, Michigan Dept of Public Health. Div of Health Assessment and Consultation, Agency for Toxic Substances and Disease Registry.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
I cant remember where i saw this (it was probobly sciencemandess a few days ago)
its so odd seeing inner lung structures on an xray
I found it quite remarkable so ill re post it
http://www.nejm.org/doi/full/10.1056/NEJM200006153422405
Figure 1 A 21-year-old dental assistant attempted suicide by injecting 10 ml (135 g) of elemental mercury (quicksilver) intravenously. She presented
to the emergency room with tachypnea, a dry cough, and bloody sputum. While breathing room air, she had a partial pressure of oxygen of 86 mm Hg. A
chest radiograph showed that the mercury was distributed in the lungs in a vascular pattern that was more pronounced at the bases. The patient was
discharged after one week, with improvement in her pulmonary symptoms. Oral chelation therapy with dimercaprol was given for nine months, until the
patient stopped the treatment; the urinary mercury level did not change during this period. At follow-up at 10 months, she was healthy, with none of
the renal, gastrointestinal, or neurologic effects that can result from the oxidation of mercury in the blood and consequent exposure of these organ
systems. The abnormalities on the chest radiograph were still apparent. Although these abnormalities are striking, the absence of clinical toxicity in
this patient illustrates the differences in the acute and chronic effects of exposure to elemental mercury, inorganic mercury (e.g., mercuric
chloride), and organic mercury (e.g., dimethylmercury). Inorganic and organic mercury are much more toxic than elemental mercury; for example, a dose
of 400 mg of mercury in the form of dimethylmercury is usually lethal.
Francisco Gutiérrez, M.D.
Lucio Leon, M.D.
Hospital Sótero Del Rio, Santiago, Chile
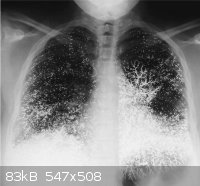
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
I just realized i saw it in this thread...
please disregard 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's some pictures of an unfortunate person's foot after exposing a cut to mercury from a light bulb. I'm not sure how much of the damage is from
mercury or if gangrene is also involved. I don't know the pedigree of these pictures so can't give any source. But they appear to be from
Caterpillar, the manufacturer of heavy construction equipment.
Attachment: Health_&_Safety_Warning_-_energy_saving_bulbs.pdf (278kB)
This file has been downloaded 545 times
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Bullshit alarm.
The pamphlet is loaded with bullshit. The very beggining describes CFLs as they're mercury rectifiers, with kilograms of liquid metal which just waits
to slosh around the house after the bulb breaks. In fact, chances are one will never see any mercury inside because the amount is tiny.
If a bulb breaks, everyone should leave the room for 15 minutes? Therefore, after 15 minutes, the danger is gone because the mercury dissapears? Just
wow.
And no, you can't get mercury poisoning by stepping on a broken bulb. You can get severe cuts which can sometimes, even if taken proper care of, end
with an infection. Standard thing.
Unbelieveable. I bet soccer moms gulp these pamphlest as they're made out of sugar. Sorry, I meant to say "natural, holistic sweeteners".
|
|
|
| Pages:
1
2 |
|