HGOPOO
Harmless

Posts: 1
Registered: 24-8-2011
Member Is Offline
Mood: No Mood
|
|
Nitromethane, Nitro methanol and Methyl nitrate !!
Hi
I want to know what is the difference between
Nitromethane, Nitromethanol and Methyl nitrate
many names for one thing ? or what ??
I want a detailed explanation please
Thanx
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
The first picture is nitromethane. It is a nitroalkane.
The second picture is nitromethanol. It is a nitroalcohol. I have never heard of nitromethanol before.
The third picture is methyl nitrate. It is an ester of methanol and nitric acid.
They are all different compounds.
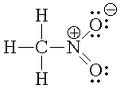 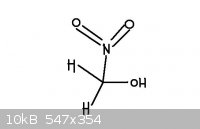 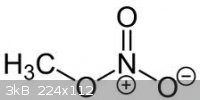
[Edited on 24-8-2011 by redox]
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The name nitromethanol is incorrect as a nitro group (NO<sub>2</sub> cannot be attached to an alcohol . . .
cannot be attached to an alcohol . . .
Methyl nitrate (CH<sub>3</sub>NO<sub>3</sub> is prepared by
nitrating methanol - in nitromethane (CH<sub>3</sub>NO<sub>2</sub> is prepared by
nitrating methanol - in nitromethane (CH<sub>3</sub>NO<sub>2</sub> , a hydrogen of methane is replaced by a nitro group! , a hydrogen of methane is replaced by a nitro group!
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
How come a nitro group cannot be on an alcohol?
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Because alcohols contain an hydroxyl group . . .
When hydrogen is replaced by nitro, the oxygen remains and a nitrate is formed!
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
Well, yeah, but does nitromethanol exist? Can a nitro group be on the same carbon as a hydroxyl? I have never heard of such a compound.
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Nitromethanol is simply a beginner's name for methyl nitrate . . .
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
No, No, that's not what I mean. I know that a nitro group on an oxygen is then nitrate. I'm asking if such a compound exists where a hydroxyl group
(OH) is on the same CARBON as a nitro group (NO2). I have never seen or heard of such a compound, but I don't see why it couldn't exist.
This picture illustrates what I am asking about.
[Edited on 24-8-2011 by redox]
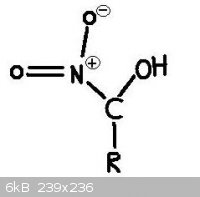
My quite small but growing Youtube Channel: http://www.youtube.com/user/RealChemLabs
Newest video: Synthesis of Chloroform
The difference between chemists and chemical engineers: Chemists use test tubes, chemical engineers use buckets. |
|
|
HellstormOP
Hazard to Self
 
Posts: 75
Registered: 13-8-2011
Member Is Offline
Mood: No Mood
|
|
Well, if you can find a way to attach a hydroxy group to a carbon atom with a nitro group on it, of course without decomposing the nitro group, then
you will have a such compound. But I know no way how that could be accomplished.
[Edited on 24-8-2011 by HellstormOP]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It is essentially not possible for both a nitro group and a hydroxyl group to be on the same carbon atom.
Basically what would happen is the hydrogen atom in the hydroxyl group would ionize off. Then the electron in the oxygen atom would migrate into the
nitro group.
The nitro group would break off as a nitrite ion, leaving an aldehyde behind. This is assuming the pH is basic. Otherwise, the nitrous acid will
oxidize the aldehyde to a carboxylic acid.
CH3-CH(NO2)OH --> CH3-CH(NO2)-O[-] H[+] --> CH3-CH=O NO2[-] H[+]
For a similar reason, chlorine atoms cannot exist on the same carbon atom as an amine group.
Although methyl nitrate is structurally similar to nitromethane, the chemical reactivity of the two compounds is quite different.
Nitromethane can undergo complex reactions in acidic or alkaline solutions. The reactions are really quite complicated, and depend on whether water is
present, or require strong heating. The reactions of nitromethane are really quite useful, but also rather obscure. Such useful products as
hydroxylamine or 2-nitroethanaloxime HON=CHCH2NO2, can result from such reactions.
In terms of chemical reactivity, methyl nitrate generally behaves like any other ester. It will hydrolse back to methanol in the presence of a strong
base or acid.
Dinitromethane exists, but methyl dinitrate is extremely unstable and does not exist at room temperature.
[Edited on 24-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by HellstormOP  | Well, if you can find a way to attach a hydroxy group to a carbon atom with a nitro group on it, of course without decomposing the nitro group, then
you will have a such compound. But I know no way how that could be accomplished.
[Edited on 24-8-2011 by HellstormOP] |
Hello Captain obvious 
Some info on "nitrometanol":
CA Index Name: Methanol, nitro-, radical ion(1-) (9CI)
Class Identifier: Radical Ion
also:
Pulse radiolytic studies of MeNO2 in alk. aq. solns. satd. with N2O show that hydroxyl radicals react rapidly with nitromethane anions. The reaction
proceeds by addn. to the C:N bond producing the transient hydroxynitromethane anion radical, i.e., OH.bul. + CH2:NO2- > CH2OHN.bul.O2-.
Source: Journal of Physical Chemistry (1968), 72(10), 3382-7
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
It's called wikipedia . . . chemicalbook . . . google . . .
|
|
|