Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Explosives from acetamide?
Hello. I am new here. I find this forum quite interesting so I registered. 
I have some ideas how to convert acetamide into explosive compounds.
Acetamide can be easily prepared at home (thermal dehydration of ammonium acetate), so I was thinking of making explosives of it.
First of my ideas was simply oxidating the NH2 group into NO2, but the product (if it exists) may be far too unstable to handle it safely.
The second idea is to add amonia on it, so the oxygen will be replaced by =NH group, which will then be nitrated (it will give compound similar to
nitroguanidine, right?). Or oxidating the acetimine's? (not sure about the name) NH2 group to NO2. It can be more stable than my first idea.
And finally - the third idea - CH3-C(NO2)=N-O-N=C(NO2)-CH3 - not sure about the name, but I think it can be: 2,6-dinitro-3,5-diazo-4-oxaheptane. If
not, please correct me. I am not good in making IUPAC names 
The preparation:
1. Acetamide + hydroxylamine (will replace the oxygen by =NOH group)
2. Dehydration of the =NOH groups, so it will join the two molecules with an =N-O-N= bond.
3. Oxidating the NH2 groups to NO2 (H2O2 + H2SO4, as usual)
The compound will probably have high VoD, density and gas volume.
What do you think? Is it possible?
[Edited on 21-9-2011 by Adas]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
First do yourself a favor and read a few textbooks on both energetic chemistry and handling procedures. Fingers and eyes come off fast and it's very
bad for the hobby in general (home chemistry). Check out "Chemistry of Power & Explosives" by Davis; it's free here for the
download.
Be exceedingly careful with your spelling, notation, & lab technique. Please read "Advanced Practical Organic Chemistry" by Casey. Lab technique
becomes almost hyper-critical with energetic materials / toxins and it's SO easy to forget some incidental little things that many people often use
the term "unstable" when in reality the material is "extremely sensitive". Even somewhat obscure peroxides can be maintained for long periods in the
proper conditions, yet can knock off fingers from static electricity that is low enough not to be felt.
I don't say any of this to diminish you interest or circumvent you from getting answers or furthering your interest. I say it because even though I do
not know you personally, a negligent injury hurts us all. Hobby & academic chemistry is in a very serious decline from many quarters. Any injury
adds fuel to the fire (no pun intended).
In regards to your question..... please give background material (where you got the concept from) authors, patents, etc. Quoting the research wherein
you got an idea is critical when asking others as they cannot do your research for you or even provide opinions unless they have a clear idea of what
you are attempting and it's relationship to a workable idea, etc.
Because of the seriousness of the subject matter, spelling notation, conventions ARE critical. There is nothing wrong with forgetting something
however the effort must be there to professionally provide enough background so that even a simplistic query is clear.
And there is NOTHING wrong with editing a post.
[Edited on 21-9-2011 by quicksilver]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Oh, sure. I am sorry, but I haven't got any materials (patents etc.). The idea just got into my head and I wanted to share.
btw, sorry for my spelling 
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Adas  |
First of my ideas was simply oxidating the NH2 group into NO2, but the product (if it exists) may be far too unstable to handle it safely.
|
The intermediate product would immediately hydrolyse with water, as fast as it formed, into acetic acid and nitrous acid.
Quote: Originally posted by Adas  |
The second idea is to add amonia on it, so the oxygen will be replaced by =NH group, which will then be nitrated (it will give compound similar to
nitroguanidine, right?). Or oxidating the acetimine's? (not sure about the name) NH2 group to NO2. It can be more stable than my first idea.
|
I have been wondering about this myself, and I do not not know if these types of reactions work so easily. Aldehydes readily condense with ammonia in
water solutions, whereas ketones require very alkaline conditions, to pull the water out, leaving behind imines. For example, acetone and ammonia will
form (CH3)2C=NH if conditions are made very strongly alkaline.
But I am not entirely sure if acetamide, CH3C(=O)NH2, would react with anhydrous NH3 to form acetamidine, CH3C(=NH)NH2. I know, however, that dry NH3
will react with ethyl acetate to form CH3C(=O)NHCH2CH3.
There are some patents dealing with the conversion of urea to guanidine, but generally strong heat and moderately high pressures (6 atm) seem to be
required.
There are numerous other reactions where ammonia can displace the oxygen, but many of these only occur through tautomeric mechanisms:
http://de.wikipedia.org/w/index.php?title=Datei:Synthesis_4-...
Quote: Originally posted by Adas  |
And finally - the third idea - CH3-C(NO2)=N-O-N=C(NO2)-CH3 - not sure about the name, but I think it can be: 2,6-dinitro-3,5-diazo-4-oxaheptane. If
not, please correct me. I am not good in making IUPAC names 
|
CH3-C(NO2)=NOH is known as ethyl nitrolic acid. There do not exist any references to it being able to dehydrate, as you suggest, and it is probably
not possible. Nitrolic acids may, however, exist as dimers, and they turn bright red in alkaline solution.
Quote: Originally posted by Adas  |
The preparation:
1. Acetamide + hydroxylamine (will replace the oxygen by =NOH group)
2. Dehydration of the =NOH groups, so it will join the two molecules with an =N-O-N= bond.
3. Oxidating the NH2 groups to NO2 (H2O2 + H2SO4, as usual)
The compound will probably have high VoD, density and gas volume.
What do you think? Is it possible?
|
The first step you propose would likely require some concentrated NaOH to drive the reaction, if the reaction is even possible.
I do not think the dehydration you describe is possible. Glyoxime, HON=CH-CH=NOH, however, can be dehydrated to furazan (5-membered ring), but this
might just be because furazan is somewhat aromatic. The dehydration, in this case, requires steam heating with KOH under pressure.
As for the third step, CH3C(=NH)NHOH would probably oxidize very easily, either by dilute H2O2, or by NO2 gas. In my opinion, it would most likely to
create acetonitrile.
CH3C(=NOH)NH2 is merely a tautomer of CH3C(=NH)NHOH. They readily convert between the different forms, with the second form, N-hydroxy acetamidine,
although I am not entirely sure which one predominates. Any oxidation would primarily be through the CH3C(=NH)NHOH form. The hydroxylamino group would
initially be oxidized to a nitroso group, and then quickly to a nitro group.
CH3C(=NH)NO2 would not be chemically stable. The oxidation is likely complex, and I am not entirely sure what the different products would be. You
might get something like:
CH3C(=NH)-N=NOH
This structure is not implausible, because HON=NOH actually has been prepared, in the form of white explosive crystals, and the compound is called
hyponitrous acid.
Usually amino groups are fairly easy to oxidize. Where you may be confused is when amino groups are in the form of salts, or when they are electron
donating, such as in the case of 2-nitro analine, they are much more difficult to oxidize.
Hope this helps.
Acetamide can be nitrated, similar to the nitration of urea, to form N-nitroacetamide. This should not be confused with the other isomer of
nitroacetamide, in which the nitro group is on the methyl group. Mention was made to this latter compound by Claus Dreier and Ole Simonsen from the
University of Odense, Denmark (1981), which melts at 82degC.
[Edited on 22-9-2011 by AndersHoveland]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Oh, thank you, Anders. I have never imagined it can be so complicated. 
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes, organic energetic chemistry can be fairly complicated, the chemistry of nitroalkanes and nitrolic acids in particular is a rather obscure subject
among chemists, which has recieved little attention in the recent literature.
I always appreciate imaginative people with energetic chemical ideas, and am more than happy to share whatever knowledge I have managed to accumulate
on the subject, to provide a basic analysis and give my own opinion about their idea or questions. 
Chemistry is a fairly complicated subject, and however much you know, even about a very specific field such as energetic chemistry, there is always
more to learn, important things to learn that you did not know about before. Things are so complex that there are not any simple rules that can
reliably be used to predict what will happen with any certainty. It takes many years of reading in the literature to be able to develop a reliable
sense of intuition about what to expect from a reaction, and even then there are many surprises. There are so many rare elements whose chemistry has
not been exhautively researched, but even the chemistry of the common elements can be surprisingly complicated, especially the higher oxidation
states. Especially in organic chemistry, the full chemistry of the nitro, nitrososo, nitramine, and oxime groups are usually not taught.
[Edited on 22-9-2011 by AndersHoveland]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | Aldehydes readily condense with ammonia in water solutions, whereas ketones require very alkaline conditions, to pull the water out, leaving behind
imines. For example, acetone and ammonia will form (CH3)2C=NH if conditions are made very strongly alkaline.
But I am not entirely sure if acetamide, CH3C(=O)NH2, would react with anhydrous NH3 to form acetamidine, CH3C(=NH)NH2. I know, however, that dry NH3
will react with ethyl acetate to form CH3C(=O)NHCH2CH3. |
To get stable imine products you need desiccants. Ethyl acetate reacts with ammonia to form acetamide and ethanol, not "CH3C(=O)NHCH2CH3."
You need to use references. You have the enthusiasm of a great chemist, but none of the diligence. Yes, it is slow and tedious to provide
references, but you keep posting inaccurate information that could cause someone to injure themselves or at least waste resources. You post so much
misinformation on obscure topics that I don't have the time to research all of it and point out all the errors, and so I'm tempted to just blindly
delete everything you post, rather than risk someone with an actual lab trusting you when you are wrong. Please don't tempt me further.
I weep at the sight of flaming acetic anhydride.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes, I know this. But I am fairly sure that I read about this sort of reaction before, involving ethyl acetate and alcholic ammonia (without any
water). I did a search, but did not find any references, unfortunately. But yes, of course reacting ammonium hydroxide with ethyl acetate results in
acetamide and ethanol. I was refering to the use of anhydrous ammonia. But perhaps I was mistaken.
I as able to find this reference, however:
"When a primary or secondary amine is heated with an ethereal salt an amide is formed and the alcohol of the ethereal salt is set free. Ethylamine and
methyl acetate, for example, yield ethylacetamide and methyl alcohol."
Journal of the Chemical Society, Volume 44, p175
I was just expressing my opinion about what I think would likely happen, these sorts of reactions are very complicated, and it is difficult to be sure
what exactly will form.
[Edited on 22-9-2011 by AndersHoveland]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I was referring to anhydrous ammonia and ammonium hydroxide.
Do not post speculation as if it were fact. If you don't find references, and it's just a stab in the dark, a gut feeling, an opinion, whatever -
don't post it.
I weep at the sight of flaming acetic anhydride.
|
|
|
Nitro-esteban
Harmless

Posts: 39
Registered: 10-4-2013
Location: Fifth dimension
Member Is Offline
Mood: inert
|
|
reacting acetamide with formaldehyde should give triacetyl hexahydro triazine which can be nitrated to RDX.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
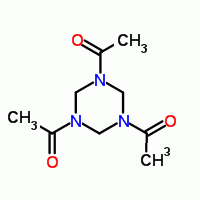
1,3,5-triacetyl-1,3,5-hexahydrotriazine
www.ingentaconnect.com/content/bpl/jlcr/1985/00000022/000000...
http://onlinelibrary.wiley.com/doi/10.1002/jlcr.2580221109/a...
_____________________________
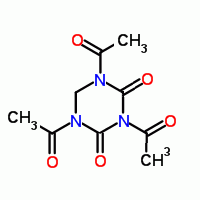
Just wondering out loud here , I wonder if 1,3,5-triacetyl-2,4-dioxo-1,3,5-hexahydrotriazine
a synthetic ' bleach activator " can lead to di keto RDX
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=18243
|
|
|
Trotsky
Hazard to Others
  
Posts: 166
Registered: 6-2-2013
Location: US
Member Is Offline
Mood: No Mood
|
|
I wonder if you could hydrolyze the carbonyl to the alcohol then nitrate. I don't know that the resulting compound would have been worth the trouble,
especially considering that simpler likely more powerful compounds are more easily had.
If you were just looking at trying to make a more complicated compound than you had previously and wanted to try a different reaction it could be
interesting, but I can think of better things to make.
|
|
|
|