| Pages:
1
..
3
4
5
6 |
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
No, mercury WILL leave residue in the flask when distilling. High purity leave little to no residue and OK purity leave quite a bit of it. My way to
characteristic purity without heavy analysis work.
All processes give more than 70%, and moss of then give 90%+ When done carefully I usually get 96% or so yield with most of theses. Thermal process
give higher yields unless it is the aqua regia one, which give the best yield and purity of all the processes.
[Edited on 3-8-2013 by plante1999]
[Edited on 3-8-2013 by plante1999]
I never asked for this.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Cool.
Can you make elemental mercury from grounded cinnabar in a steel reactor heated over 600C with air pumped into? This would be the easiest way to
procceed, and I read that there will be formed SO2 and elemental mercury in vapor form, which will condense and accumulate in the collecting flask.
Is mercury compatible with plain steel, carbon steel and 300 series stainless?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
All yes, buy did you even read lab scale industrial process? And no, it would definitly not be the "easiest" way to proceed, you should read my
resume.
I never asked for this.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
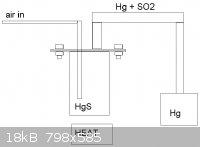
Cool, I've got to check that. I aint got no much glassware, just basic distillation, separation, refluxing and filtering apparatus, mut I prefer to
make my own equipment. For mercury reactor I just need to take 4" steel tube, weld a bottom to it and make the flange neck and flange plate and weld
the condenser and air inlet for it, and its ready. 600C is easily reaced with normal propane burner, an air injected carbon bed would be uneccessarily
powerful and a coke bed could even melt the reactor. Please see the picture.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | | No, mercury WILL leave residue in the flask when distilling. High purity leave little to no residue and OK purity leave quite a bit of it.
|
OK thanks plante. Basically BP determination on a single distillation. Very good yields possible.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
This is an example of an amateur gold refining retort, used for separating gold from the amalgam. I assume that it could be used to separate sulfur
and mercury. Testimento's "cup" design is much better than the one in the video, as the heat only passes through one surface.
http://www.youtube.com/watch?v=ShgM07O1rqQ
The use of a water trap might serve to clean the mercury that condenses . . . the water would form an acidic environment in the presence of SO2, and
would help to clean the mercury. In my opinion, a two-stage steel condenser is the way to go, with the first stage being air-cooled and the second
stage being water-cooled to condense any remaining mercury.
The gold rush (personified here) used massive amounts of mercury to extract gold. The ore would be crushed and shaken along copper sheets coated in
mercury. The amalgam would then be heated to drive off the mercury, (usually) condensing in a tub of water.
The Spanish used to send criminals to work in cinnabar mines. Mercury is no joking matter, be very careful.
[Edited on 17-10-2013 by Awesomeness]
[Edited on 17-10-2013 by Awesomeness]
Fear is what you get when caution wasn't enough.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I just managed to buy 2kg of cinnabar dirrectly from china with the money donated recently and the personnal funds I had, in a month or so it should
be here, and I will be able to do the final work, pictures, all the processes description and purification.
I also managed to buy a cheap chinese 10g 0.001g scale, so calculation should be quite precise.
I never asked for this.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I experienced with a new cinnabar type, not the usual granular mineral, it was a fine dust. Many problem arrised from that, but I have a few general
recommendation to make before I thoroughly analyse the inconvenient of fine cinnabar, and it advantages..
In the sulphur/sodium hydroxide/aluminium process, there is serious problem if you lack of hydroxide ions in solution. It appear that if there is not
enough hydroxide ion per sulphide, the mercury precipitate back... As black sulphide. It appear that the acidic condition precipitate the dissolved
mercury. I have more research onto that. Secondly, Nitric acid do leach cinnabar very effectively when copper is added to generate NOx that
aggressively oxidize the sulphide. However, if you want liquid mercury, you should calculate some excess and use a stochiometry based onto the fact
that copper is a reactant too, because copper dissolve preferably in nitric acid then reducing the mercury solution. In my experience, the excess is
sort of hard to calculate due to the many side reactions happening. After 2 or three day, the copper started to amalgamate, meaning the nitric acid
had been consumed. The mercury by this method is impressively pure.
Aggressive HNO3/NOx leach based on nitrite + nitric acid + cinnabar yielded on cooling a white crystalline solid which I have to believe is mercury II
sulphate.
Here is a reaction I hypothesized based onto mys results, take note there is certainly other side reactions.
14HNO3 + 3HgS -> 3Hg(NO3)2 + 3H2SO4 + 8NO + 4H2O
10HNO3 + HgS -> Hg(NO3)2 + H2SO4 + 8NO2 + 4H2O
8HNO3 + 3Cu -> 3Cu(NO3)2 + 2NO + 4H2O
4HNO3 + Cu -> Cu(NO3)2 + 2NO2 + 2H2O
Cu(NO3)2 + H2SO4 -> CuSO4 + 2HNO3
Hg(NO3)2 + H2SO4 -> HgSO4 + 2HNO3
I never asked for this.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Obviously some sort of verification should be done. The mercuric nitrate/sulfide complex of yesteryear isn't ruled out.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Yes, of course it should be analysed, although in such conditions I doubt any sulphide persist, really.
I never asked for this.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
BE WARN BE WARN!!!
Aliexpress seller bian sell fake cinnabar. The cinnabar I worked on in the last few days was not in fact cinnabar, at least very very very far from
pure. I should always rely to the classical mineralogical test!
The open tube showed it, it contain much much less then a 1% Hg, the remainder being white mineral ash that is not silica, and a red dye. Don't get
scammed, I just got scammed really really hard, 150$ for nothing. Buy from gaofudev on ebay, he is a legit seller.
I never asked for this.
|
|
|
bfesser
|
Thread Split
2-1-2014 at 07:19 |
jharmon12
Harmless

Posts: 48
Registered: 27-2-2013
Member Is Offline
Mood: No Mood
|
|
Quick question about buying cinnabar from gaofudev on ebay. I looked at his listings and he has high quality stuff, but you can buy mercury by the
pound online for $186.00, purified. I know I saw it less than a year ago for $150.00/lb on another site, but can't find it right now. This guy
gaofudev charges so much for his cinnabar, if you are going to just retort mercury to get mercury, why not just buy it from online at a place like:
http://www.sciencecompany.com/-P16860.aspx?utm_source=google... ?
I am trying to figure out the reasoning here. Maybe I am not looking at a cheap enough sample on ebay? Where is the cheapest place to get cinnabar
chunks or powder???
Thanks,
Joel
|
|
|
jharmon12
Harmless

Posts: 48
Registered: 27-2-2013
Member Is Offline
Mood: No Mood
|
|
Found it on Amazon from GalliumSource:
http://www.amazon.com/GalliumSource-LLC-Liquid-Mercury-454g/...
Less than $100 and free shipping. How cheap can we get cinnabar to save money making mercury? $100/lb seems pretty cheap.
Joel
|
|
|
jharmon12
Harmless

Posts: 48
Registered: 27-2-2013
Member Is Offline
Mood: No Mood
|
|
What about a shotgun recoil reducer. 1 lb of mercury for $43.00: http://mpcsports.com/stockmodel78x516oz.aspx
Joel
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
"why not just buy it from online at a place like: http://www.sciencecompany.com/-P16860.aspx?utm_source=google... ?"
I don't live in the US...
I doubt the recoil contain mercury, and if so, probably less then a pound.
The point is to buy material which is not hazardous, proceed it, which is fun, and store it. Although it is nice to make it cheaply, it is not exactly
the point.
I never asked for this.
|
|
|
khlor
Hazard to Self
 
Posts: 82
Registered: 4-1-2014
Location: Who knows, really...
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | <div align="center"><img src="../scipics/_warn.png" /> <em>Mercury and it's compounds are <a
href="http://en.wikipedia.org/wiki/Mercury_poisoning" target="_blank">cumulative neurotoxins</a> <img src="../scipics/_wiki.png" /> and
<a href="http://en.wikipedia.org/wiki/Mercury_cycle" target="_blank">persistent environmental pollutants</a> <img
src="../scipics/_wiki.png" />! Proceed at your own risk & <u>dispose of wastes responsibly</u>.</em> <img
src="../scipics/_warn.png" /></div><br /><hr width="80%" />
I know that oxidizing cinnabar HgS make mercury and SO<sub>2</sub> but does somebody know a leach for leaching mercury out of it and
precipitate the mercury? I want mercury to make sodium hydroxide from electrolisis and disolve gold plating...
Thanks!!!!
Please check my website in the signature!!!
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: subscript;
added warning]
[Edited on 11.10.13 by bfesser] |
you don't exactaly need mercury to this. you could use a diaphragma cell, I tryed using paper filter(those you use to make coffee) and worked. sodium
hydroxide in one side of the cell, well... at least to the sodium hydroxide.
[Edited on 5-1-2014 by khlor]
|
|
|
jharmon12
Harmless

Posts: 48
Registered: 27-2-2013
Member Is Offline
Mood: No Mood
|
|
My mercury experiment
Hello.
Well, I tried to extract mercury from an ore sample sent to me from South America with an unknown vermillion content. I used the chemical process
laid out by Neptunium in the "Mercury from Cinnabar" thread. I think there is so much other rock in the ore that I cannot separate it from the
mercury that is likely present.
I know that using extreme heat and airflow is an option when processing cinnabar, but I was trying to figure out how the chemical process works first.
I started with 40 Ml of water and added about 10 grams of sodium hydroxide and 3 grams of sulfur and allowed it to turn into an orange-red liquid with
heat and continuous stirring.
I then added 10 grams of cinnabar (ore) to the mix after it cooled, and allowed it to mix with a magnetic stirrer until it cooled off.
I added aluminum, 20 mesh flakes, until the heat and gas liberation was mostly done. After cooling, I didn't have any permanganate as recommended by
Neptunium, so I added potassium dichromate in solution, as my understanding from the original thread was that this could also be used.
I am frustrated. The problem I am having is that Neptunium's description is so cryptic on how he "cleaned" the aluminum/mercury alloy, that I am not
sure on how to proceed. He wrote a couple of sentences on using permanganate to clean it up and then using nitric acid, then finally some
hydrochloric acid. But I cannot make heads or tails of what the details of this process might be.
So far, I have a 100 mL watery solution of what looks like black filth with some K2Cr2O7 added to it.
I sent a message directly to Neptunium and Platte, who is also involved with mercury experiments, but they are not responding and I want to continue
with this project.
I would appreciate any feedback on how to proceed to separate the mercury from the rock dust I currently have in solution.
Thanks for any help you might be able to offer...
Regards,
Joel
|
|
|
bfesser
|
Threads Merged
7-1-2014 at 18:39 |
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I'm not answering eh...
You will now know from now on what is not answering, and my username is plante by the way.
I never asked for this.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Neptuniums post is not cryptic at all. To clean amalgam you first need to have amalgam. Do you have amalgam?
Give a better idea of what type of mineral you are using, with zero description is it difficult to suggest a method to separate the vermillion/mercury
from other components. Perhaps a photo of it? Also, if the amount of mercury containing mineral is unknown, are you sure it contains any and if so the
amount might be so small that the method you are using is simply impractical.
Just a note; sometimes giving people time to respond to your U2U messages is worth the wait. Patience... Saying they aren't responding quickly enough
to your liking in the thread is a tad inappropriate.
|
|
|
jharmon12
Harmless

Posts: 48
Registered: 27-2-2013
Member Is Offline
Mood: No Mood
|
|
Hi Mailinmypocket.
This ore is coming from a mine that extracts cinnabar, orpiment, and realgar. It was sent by someone that is working the mine and has access to the
ore. The ore clearly has dots of red crystals of what appears to be vermillion throughout its matrix.
I followed Neptunium's description up to the point of adding the aluminum and I added it until the heat and gas release was minimal. From what I
understand from reading Neptunium's description, adding the aluminum powder should have created an amalgam of mercury and aluminum. So, what I have
is whatever the non-mercury ore content was, plus an amalgam of mercury/aluminum. So, the trick is going to be separating the two.
I am not sure a picture would do any justice, as what I have is simply a black "sludge" that has sunk to the bottom of the beaker in the water/K2Cr2O7
solution.
The issue, I think, is that all the experiments listed in this thread so far involved a known quantity of mercury, such as the dental fillings by
garage chemist...or a very good sample of vermillion that yielded almost 100%, by weight, of mercury, as illustrated by Neptunium and Plante
(accidentally forgot your name spelling earlier...my bad). My experiment involves the use of ore with an unknown quantity of vermillion in it.
Furthermore, the content of the rest of the ore is unknown. Perhaps it is granite? I do not know.
I have the option of getting 20 more lbs of this ore, as a granulated powder, for $13/lb, but don't want to commit to this until I test this sample
first to see how much mercury I can "squeeze" out of it. If it appears that I can get a decent amount of mercury out of it (probably around 50% or
more), I will consider it worth it to buy all 20 lbs and use that as my personal mercury source going forward. Otherwise, I will just get my mercury
in 1 lb quantities from Amazon.
So, that gives you a picture of what I am trying to accomplish and what the end goal is. Thanks for the help so far.
Regards,
Joel
[Edited on 8-1-2014 by jharmon12]
[Edited on 8-1-2014 by jharmon12]
|
|
|
jsc
Hazard to Self
 
Posts: 65
Registered: 16-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  |
A Castner cell doesn't use mercury, it electrolyses molten NaOH. Member 'len1' constructed one of these and reported extensively about it on this
board (search for it). 'Not in a miliion years'? Who is sounding dumb now, eh?
[Edited on 24-12-2011 by blogfast25] |
By "Castner cell" I am referring to the cells used in the Castner-Kellner process which uses mercury.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Those cells can be super easy to make if you have enough mercury and some use for chlorine in salt water.
The site is called sciencemadness.
http://www.youtube.com/watch?v=xiqmEibSY0I
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
I hope we are not about to lose the cinnabar thread due to spam being appended to the end.
(Will this save it?)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Not to worry...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by tibow64  |
I suspect that the fact that there seem to be no youtube vids demontrating this possibly provides an answer to your question, though!
|
This has to be the dumbest thing I have heard in a while. It is not because no one made a youtube video that no one tried it. Most things are posted
on forum, or are not posted on the internet at all when it comes to home chemistry.
BTW, I already burned cinnabar in a quartz tube with air flow, it works very well to yield mercury.
[Edited on 6-1-2015 by plante1999]
|
|
|
| Pages:
1
..
3
4
5
6 |