Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
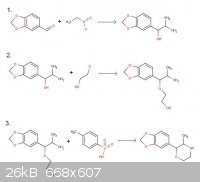
3,4-MD-Phenmetrazine
According to Journal fur praktische Chemie 4. Reihe. Band 21. 1963 pp 12-17
1. Benzaldehyde + nitroethane --> phenylpropanolamine
2. Phenylpropanolamine + 2-chloroethanol --> ether ethanol material
3. Ether ethanol + toluenesulfonic acid (-H2O) --> Phenmetrazine
To my innocent eyes, this seems like a perfectly practical (gentle) route for making 3,4-methylendioxyphenmetrazine starting with piperonal. Please
see the attached scheme.
Opinions on whether this would work or not would be greatly appreciated!
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Wheres your references for these reactions and wheres your reference that morpholine stimulants will even be metabolized in a similar fashion to
amphetamines. The ring is going to distort the configuration meaning it may not bind with the same characteristics that you are looking for in this
substitution.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
The reactions 2 and 3, starting with phenylpropanolamine, are outlined in the German journal that I cited.
The synthesis of phenylpropanolamine from benzaldehyde and nitroethane is well known.
As for pharmacological action, my thinking is that most ring substituted amphetamines (usually psychedelic in nature) lose activity when N-methylated.
For example TMA-2 is active, TMMA-2 is not. But the only two known to retain activity are MDMA and plain old MA. Well, here with phenmetrazine we
essentially have MA with the N-methyl looped back around to make a ring using another carbon and an oxygen.
Logic: Whereas MA is more euphoric than A, phenmetrazine is reportedly more euphoric than MA. Whereas MDMA is more euphoric than MDA, might
MD-phenmetrazine be more euphoric still?
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Your reaction scheme won't work. The amine functionality is much more nucleophilic than the alcohol and thus the chloroethanol will alkylate that.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
The point I'm trying to make to you is the pharmacology of the two are highly different. Aminorex which this seems like it would share much similarity
to does not respond to the same substitutions that Amphetamines do meaning this would more then likely be a let down.
Odds are you would be better off to experiment with the 4-floro derivative here or better yet read up and gain a better understanding about the
compound you are trying to synthesize so that you don't waste your time on futile efforts.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
Keep in mind that the benzaldehyde to ppa route involves two steps forming the nitro alcohol intermediate with benzaldehyde nitroethane and
triethylamine and then hydrogenating the nitro alcohol to yield ppa. To find a detailed example search Google patents for a patent thats purpose is to
claim a method of separating d and l amphetamine.
|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
BTW if your interested in dopamine agonists. Check out the patent ..NOVEL BENZAZAEPINES.. in one example they use d-amphetamine as a reagent.
|
|
|
Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
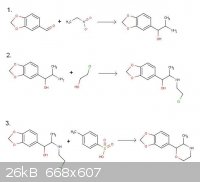
Vulture, I'm sorry, you're correct. The 2-chloroethanol will certainly alkylate the amine group. I was very tired when I drew that up. Here's the
corrected drawing.
Here's the German paper:
http://www.scribd.com/fullscreen/76796247?access_key=key-1ff...
Sedit: Interesting that you mention the aminorex class. Alexander Shulgin was found to have written personal notes to the effect that he intended to
synthesize the 4-methylaminorex equivalent of MDMA. Sadly, he never got around to it and now probably never will.
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
I hate to be the bearer of bad news, but your new reaction schemes have a few problems still. Take a look at your alkylation step.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Shrulgin may not have but or russian friends over hyperlab have IIRC and they where less then impressed. The structural orientation of the ring will
dramaticly effect binding and metabolic properties of a compound. Look deeper into this class of compounds to see if there is any chance of this being
effective.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dope Amine  | | Logic: Whereas MA is more euphoric than A, phenmetrazine is reportedly more euphoric than MA. Whereas MDMA is more euphoric than MDA, might
MD-phenmetrazine be more euphoric still? |
Your logic does not sound like logic to me. You fail already at the axiom. Since when is a property such as "euphoric" something that can be
correlated with structure between compounds having different modes of action? That sounds like science fiction rather than science.
Next, who told you that MDMA is more euphoric than MDA? And the rest of comparisons? Do you have any references for any of that or just some anecdotal
recounts?
Finally, why on earth would you resort to some weird esoteric logic when there is a structure-activity theory (SAR) available for all of the mentioned
classes of compounds? Your approach is like planing to cross the Atlantic with a canoe boat when you can just take a jet instead. My advice is to
study medicinal chemistry rather than resorting to structural masturbation.
I don't really understand what you are up to, but just evaluating a couple of SAR's on your target, makes it appear as poor choice for any kind of
activity, let alone something as subjective as promising euphoric property and some undefined activity.
The 5-HT2A affinity decreases dramatically in the isopropylamine to morpholine side chain transformation (e.g., the Ki drops by 200-fold from
4-bromo-2,5-dimethoxyamphetamine to 2-(4-bromo-2,5-dimethoxyphenyl)morpholine and intrinsic efficacy goes to essentially nothing; see DOI:
10.1021/jm040082s for details). You can therefore be pretty sure that 3,4-methylenedioxyphenmetrazine would totally lose even that tinny amount of
5-HT2A mediated activity that MDMA has (and which makes MDA such a medicinally valuable hybrid).
In the domain of SERT mediated activity, the 3,4-methylenedioxy substitution is well known of not being transferable between different structural
classes of mixed monoamine releasers. For example, the 3,4-methylenedioxypyrovalerone is just a dull stimulant. Obviously no SERT mediated activity at
all. So, as you see, even inside the same mode of action, the structural features don't correlate. As far as I know from literature examples, the only
methylenedioxy bioisostere that was ever found transferable at least between two structural classes of mixed monoamine releasers is the 3,4-condensed
benzene ring. On the other hand, in the more selective monoamine releasers of dopamine and noradrenaline, the 3,4-dichloro substitution pattern is
relatively well transferable between several structural classes. Yet, to my knowledge none of the corresponding phenmetrazine-like compounds, the
2-(2-naphthyl)morpholines or 2-(3,4-dichlorophenyl)morpholines, have ever been reported in the literature.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Hello. I want to thank everyone for their responses, thoughts and criticisms. I'm glad to see that chemical discussion is quite active as well as
informed in these forums.
I certainly admit that I did not look into the literature for SAR info. I had always been more concerned with whether it could be made easily. I'm
more informed when it comes to organic chem than pharm.
Fair enough, that this compound doesn't seem to hold much promise. MDMA IS more euphoric than MDA, just like MA is more euphoric than A. And
Phenmetrazine IS more euphoric than MA. Ki's be damned.
Oh, and here's that fixed reaction scheme. I feel silly...
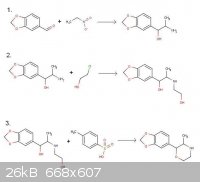
[Edited on 31-12-2011 by Dope Amine]
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
in the aminorex class methoxylation only led to dampened stimulant (more like antidepressant properties) i would expect something similar to
extrapolate out to the phenyl morpholine class.
Give me librium or give me meth!
Patrick Henry....
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Methylenedioxy-N,N-dimethyl tryptamine was reported to have zero activity, while DMT is the "most intense" hallucinogen.
Methylene dioxy bridge is very subtle, and SAR is very complicated - adding additional geometries or removing them usually does not result in an
addition or removal (or even vice-versa) relationship to activity.
If it were that easy, it would have been made already.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | As far as I know from literature examples, the only methylenedioxy bioisostere that was ever found transferable at least between two structural
classes of mixed monoamine releasers is the 3,4-condensed benzene ring. On the other hand, in the more selective monoamine releasers of dopamine and
noradrenaline, the 3,4-dichloro substitution pattern is relatively well transferable between several structural classes. Yet, to my knowledge none of
the corresponding phenmetrazine-like compounds, the 2-(2-naphthyl)morpholines or 2-(3,4-dichlorophenyl)morpholines, have ever been reported in the
literature. |
The 2-(2-naphthyl)morpholines or 2-(3,4-dichlorophenyl)morpholines have just been reported by Rothman et al. in WO2011146850. As it turns out, the
3,4-benzo system induces 5-HT uptake inhibition in the phenmetrazine structural class (EC50 = 105 nM). And not only that, the 3,4-dichloro also does
so. Some of the tested compounds also have a remarkable 5-HT release activity.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Sedit: Interesting that you mention the aminorex class. Alexander Shulgin was found to have written personal notes to the effect that he intended to
synthesize the 4-methylaminorex equivalent of MDMA. Sadly, he never got around to it and now probably never will.
I had this discussion with him and he thought the aminorex would be "very interesting." Years later I was getting ready to make it and found it had
been synthesized, tried and found to be anything but interesting. I will try to run that ref down tomorrow. The mechanism of actions is apparently
quite different from aphetamines.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
edgeofacliff
Harmless

Posts: 30
Registered: 4-6-2012
Member Is Offline
Mood: No Mood
|
|
"without resorting to structural masturbation" that on cracked me up.
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
And don't forget the possible risk of acquiring PPH with any of the aminorex analogs. Even taking Viagra for the rest of your life is no cure.
|
|
|
nexthiroshima
Harmless

Posts: 1
Registered: 6-8-2013
Member Is Offline
Mood: No Mood
|
|
here's a video on synthesizing 2-chloroethanol from ethylene glycol and HCL.
http://www.youtube.com/watch?v=bKiXxoRZ_VQ
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
If you are interested in 5HT2A activity, check out "Molecular Pharmacology, December 2006 vol. 70 no. 6 (1956-1964)"
For the N-benzyl phenethylamine analogues, the selective IPI3 pathway activation of the 5HT2A receptor seems to be mediated by two phenylalanine
residues (339 and 340). The article demonstrates that mutants have dramatically reduced activity. It appears that the binding is mediated by pi-pi
interactions between the two aromatic moieties (the benzyl ring on the ligand and the ring on the Phe339/340 residues).
Curious to me is why the 2-methoxy substitution on the benzyl ring (of the phenethylamine ligand) so dramatically increases the Ki and intrinsic
activity.
Of course, this does not address the SERT binding. I'm not sure what kind of SAR studies have been done on that particular protein.
|
|
|