Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Addition of acids to aldehydes and ketones. Possible?
Hello SM,
I was searching on Google, but couldn't find any info about this. I think it would be an useful reaction in some cases. I think it needs a special
catalyst, if the reaction can proceed, but I don't think it's impossible. What do you think about this?
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not understand what you are asking. Addition of acid to aldehydes or ketones tends to shift the equilibrium to the formation of tautomers, that
tend to be more reactive/vulnerable to oxidation. This thread will soon be moved to "beginnings"...
[Edited on 5-1-2012 by AndersHoveland]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
AndersHoveland
| Quote: |
I do not understand what you are asking. Addition of acid to aldehydes or ketones tends to shift the equilibrium to the formation of tautomers, that
tend to be more reactive/vulnerable to oxidation. This thread will soon be moved to "beginnings"...
|
As usual, this statement is not really true. IMHO this forum is not just about regurgitating facts (or fiction) - especially considering a beginning
organic textbook is not even close to comprehensive - but to help members learn to utilize the knowledge they do possess. In this regard, I would
recommend you purchase an intermediate level organic text, and perhaps refrain from commenting when you haven't much to contribute.
Adas
To answer the question, and highlight my point about utilizing knowledge (I presume you are familiar with pKas):
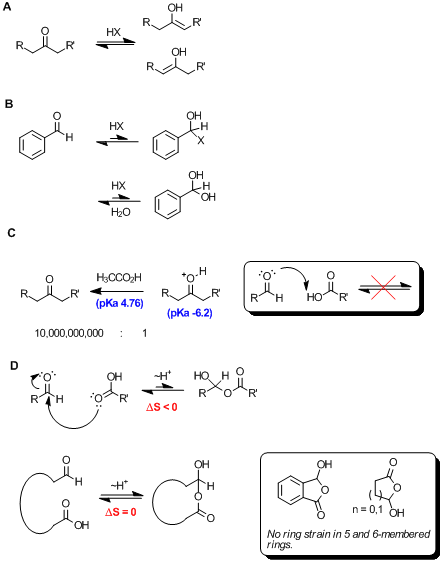
We all know that strong acids, such as mineral acids, can catalyze tautomerization of enolizable ketones and aldehydes (Scheme A),
and that this often leads to decomoposition of the aldehyde due to self aldol reactivity. In cases where the carbonyl compound cannot tautomerize,
such as benzaldehyde, mineral acid can lead to a halohemiacetal (Scheme B), though this is not a stable species, and thus
the equilibrium lies to the left. If the bulk solvent is water, mineral acid can catalyze the hydration to the hydrate shown, and while more stable -
that is to say hydroxide is a poorer leaving group than chloride - this hydrate is prone to undergo the Cannizzaro reaction.
I presume, however, that Adas is referring to carboxylic acids adding to carbonyl compounds. Given the very large pKa difference between a carboxylic
acid (e.g. acetic acid pKa 4.76, memorize this!!) and a protonated ketone (pKa -6.2), this reaction would be considered not to take
place. In cases where pKas are >4 units apart, it's not appropriate to invoke acid or base catalysis. See Scheme C
What about a carboxylic acid (relatively poor nucleophile) or carboxylate (slightly better) adding to an aldehyde (very good electrophile)? This is
certainly possible, and while likely an exothermic reaction due to the formation of a C-O sigma bond, it's realistically unfavorable due to the
decrease in entropy as the two starting components become one in the product. This equilibrium (Scheme D) likely lies to the left.
However, in cases where there is no change in entropy, the outcome is different. This scenario arises in intramolecular reactions (Scheme
D), and is uninhibited when the reaction forms a thermodynamically favorable 5- or 6-membered ring. This reaction is certainly favorable,
and the semi-anhydride product is the only form observed by NMR (i.e. >20:1 ratio favoring semi-anhydride).
Sources cited
Evans pKa Table
[Edited on 5-1-2012 by Arrhenius]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Oh, thanks, Arrhenius, this helped me a lot. Though I don't understand really everything, because I am just 16yrs old, but you still explained many
things to me.  
Rest In Pieces!
|
|
|
|