CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
Making a gas bubbler
I need to be able to make hydrohalic acids of exact concentrations (i.e. 32% HCl, 55% HBr etc.) and from reading threads on this forum, I've concluded
that the way to go is to pour 98% H2SO4 onto dry NaCl and lead the HCl gas, via a tube, into water. I found a good thread here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
in which peach posts his/her contrivance. I'm very low on money at the moment and was hoping I could just make do with what I've got. Heres what I'm
thinking:

I know this is a caveman apparatus but I'm broke at the moment so I have to McGyver the things I have at hand. My idea is to put the NaCl at the
bottom of the bottle then pour 98% H2SO4 in and rapidly stopper the bottle.
My major concern is that HCl gas will escape before I have a chance to stopper the bottle. However, it won't form instantly so I should have a good
second or two before gas builds up in the bottle. Are there any reasons why this simple apparatus would not work? Also, this metathesis reaction very
exothermic? I'm thinking of using a borosilicate flask instead of the bottle, that way I can add the H2SO4 in through a claisin head but I don't have
any quickfit addition funnels unfortunately.
EDIT: I have some narrow PVC tubing but the most convenient tubing to use would be this bunsen burner tubing:

due to its flexibility. Every bunsen burner I've seen in my life, had this same tubing (the same color and all) so I'm guessing everyone here is
familiar with it. Is it made of natural rubber?
[Edited on 26-2-2012 by CrimpJiggler]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
A gas bubbler needs to be protected against suckback, which would otherwise be inevitable if you are trying to dissolve something like HCl, which is
violently absorbed by water and will create a vacuum in the gas production flask. Use two gas bubblers in series, with the first one connected in
reverse!
In order to capture all the gas from the reaction, you simply use a pressure-equalized dropping funnel to add the acid to the salt when the entire
apparatus is already set up and everything connected.
And if you need acid of exact concentration, you absolutely have to titrate it with standardized NaOH solution!
Is there a reason why you can't just titrate store-bought HCl and write the exact concentration on the bottle?
I did so with my bottles of reagent grade "98%" H2SO4, which all turned out to be exactly 96%. I had to know the exact concentration for the
preparation of oleum from SO3 and H2SO4, in which small uncertainties in the water content of the H2SO4 can cause a large error in the final oleum
concentration. It's good to know for sure how good your reagents are!
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
I thought that the inverted funnel would prevent water from being sucked back due to the large amount of water that would have to fill the funnel
before any of it could reach the tube. Does the air get sucked out of the reaction flask along with the HCl? Anyhow, I know you're talking from
experience so I'll take your advise and add a second bubbler to the apparatus. I'm not entirely sure what you mean though. I'll draw another diagram
of what I have in mind.
I agree, I've concluded that I'm going to have to start titrating to determine the concentration of my acids. I wish I had some phenolphthalein, it
will be a real pain in the ass using pH strips to determine the endpoint. Then again, I can just do a series of rough titrations to narrow it down.
What do you mean about store bought HCl? In my country, we don't have muriatic acid, all the HCl drain cleaners here seem to have a max concentration
of 10% and contain surfactants.
EDIT: When you say to use two gas bubblers in series, are you referring to something like this?

[Edited on 26-2-2012 by CrimpJiggler]
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
I don't know if this help,
here is myst32YT's video on how to catch highly water soluble gas:
http://youtu.be/kqQSpRus-t0
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
Thats greatly helpful, thanks. Now that I think of it, I actually have one of those quickfit joints with a tubing inlet. I knew that thing would come
in useful.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
CrimpJiggler, no, you didn't understand what I was saying.
You take your gas generator, connect an empty gas washing bottle in reverse, and then your gas washing bottle filled with water or other absorption
liquid!
Look at the picture I attached. The gas stream goes from the right side to the left. See the two gas washing bottles on the left side of the
"Probengefäß"? That's how I meant it. The first bottle (the right one) is empty and connected in reverse, and the second bottle (the left one)
contains your absorption liquid (NaOH in the picture).
If the liquid in the second bottle gets sucked back, it goes into the first one, where it is then pushed back into the second one through the hose.
The inverted funnel method from the video is also a possible solution and very suitable for the beginner, but the disadvantage is that the exiting
gases cannot be further used and escape into the atmosphere. With gas washing bottles, you can connect several in series or use ones with sintered
glass bubbler for more efficient absorption of gases that aren't so soluble, or from gas streams that contain the desired gas only at low
concentration.
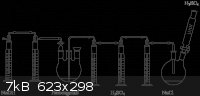
[Edited on 26-2-2012 by garage chemist]
[Edited on 26-2-2012 by garage chemist]
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
Does much of the gas escape? That was some heavy bubbling at the start of the reaction in that video, I wonder what percentage of the HCl escaped the
beaker before being dissolved. I'm going to use the inverted funnel method since I'm a beginner and have all the equipment I need but in the future
I'm gonna get a proper setup so I can work with gases efficiently.
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
A quick side question: does the metathesis reaction between H2SO4 and NaCl yield NaHSO4 or Na2SO4? In other words, should I add the reactants in a 1:1
or 1:2 ratio.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
You need to scrub the gas thouroughly and then make sure the water won't be pushed in by the atmosphere when the gas dissolves in the water. There's
an old, textbook procedure for avoiding that - placing the funnel few milimeters above the solvent. Nothing will escape, trust me - hydrogen chloride
is violently soluble in water. When the concentration of HCl in the water climbs above ~20%, you can lower the funnel onto the surface of the water
(like in the video done by myst32YT).
A suckback bottle is not required if you follow these instructions. It wastes hydrogen chloride and is just one more piece of glassware to burst if a
too low pressure develops.
Oh yes, use ice cold water. You can actually use a distilled water ice slush for the solvent, and when the ice starts melting, which
will happen quickly because of the heat released, immerse the vessel into a tap water ice slush.
Make sure the acid is always very cold because that way you could push it to 40%. It should be stored in a leak tight, sturdy bottle, of course. If
opened at room temperature, it will expell excess HCl which is quite dangerous. I'm not sure, but it might even fizz. Be careful.
garage chemist, the setup you made is extremely expensive and would require lots of reagents. An inverted funnel is a method old as hell and it
works fine enough. It's not hydrogen cyanide. Nothing will leak in dangerous amounts if he's careful.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
A regular old distillation apparatus could be used with no water in the jacket and nothing in the receiving flask, then rubber tubing could be
attached to the vacuum inlet and attached to the inverted funnel. Assuming all that is available..
[Edited on 2-26-2012 by AirCowPeaCock]
BOLD
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
For small setups, scrubbing can be done with a 50 cm long, 1 cm diameter glass tube packed with granulated calcium chloride. It traps water, and the
sheer length and the large area packed in such tight space will stop any small quantities of aerosol of sodium chloride that might be released.
After you're done, plug the tube tightly and you can use it again. I doubt your experiment will consume even a tenth of the desiccant.
There's no need for complicating this. It's a neat little procedure that can produce small quantities of very concentrated acid.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Endimion, that setup isn't something that I would actually build, it's just a picture that I borrowed from Lambdasyn to illustrate what I was saying.
Also, only two of those washing bottles are filled with something (the ones with "NaOH" and "H2SO4" written below them), the other ones are empty
safety bottles. It's a setup to gas a solution in the flask with anhydrous HCl gas, with absorption of the excess HCl in NaOH.
If I had to absorb a gas in water, or wash a gas with a liquid then I would simply use one of my special Normag gas washing bottles, these work in
both directions and are suckback-proof by design. I'll make a picture of it.
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
I setup the apparatus in the video and gave it a test run. I didn't have any beakers big enough for the funnel so I used a pyrex measuring jug. Due to
the slanted walls of the pyrex jug, the gravity didn't put enough force on the water so the gas forced all the water out of the funnel and
consequently, most of the gas escaped. All that HCl is going to wreak havoc on my retort stands. I could smell some H2S for some reason. I could
probably even get it to work with a pyrex jug, I'd just have to submerge the funnel a bit deeper. In fact, thats probably what my major mistake was, I
had trouble seeing the clear funnel through the jug so I probably didn't submerge it enough.
Quote: Originally posted by Endimion17  | | You need to scrub the gas thouroughly and then make sure the water won't be pushed in by the atmosphere when the gas dissolves in the water. There's
an old, textbook procedure for avoiding that - placing the funnel few milimeters above the solvent. Nothing will escape, trust me - hydrogen chloride
is violently soluble in water. When the concentration of HCl in the water climbs above ~20%, you can lower the funnel onto the surface of the water
(like in the video done by myst32YT). |
Really? Place the funnel "above" the solvent? Are you saying that the HCl that comes out of the funnel is more likely to dissolve in the water than it
is to escape into the air? What is scrubbing? I've heard the term a million times but I never got around to finding out what it is exactly.
[Edited on 26-2-2012 by CrimpJiggler]
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Have you ever seen the ammonia fountain demonstration? Well it would work with Hydrogen Chloride too.
BOLD
|
|
|
resveratrol
Hazard to Self
 
Posts: 65
Registered: 6-11-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AirCowPeaCock  | A regular old distillation apparatus could be used with no water in the jacket and nothing in the receiving flask, then rubber tubing could be
attached to the vacuum inlet and attached to the inverted funnel. Assuming all that is available..
[Edited on 2-26-2012 by AirCowPeaCock] |
That's a good idea.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by garage chemist  | Endimion, that setup isn't something that I would actually build, it's just a picture that I borrowed from Lambdasyn to illustrate what I was saying.
Also, only two of those washing bottles are filled with something (the ones with "NaOH" and "H2SO4" written below them), the other ones are empty
safety bottles. It's a setup to gas a solution in the flask with anhydrous HCl gas, with absorption of the excess HCl in NaOH.
If I had to absorb a gas in water, or wash a gas with a liquid then I would simply use one of my special Normag gas washing bottles, these work in
both directions and are suckback-proof by design. I'll make a picture of it. |
Oh, that settles it, then.
Quote: Originally posted by CrimpJiggler  | Really? Place the funnel "above" the solvent? Are you saying that the HCl that comes out of the funnel is more likely to dissolve in the water than it
is to escape into the air? What is scrubbing? I've heard the term a million times but I never got around to finding out what it is exactly.
[Edited on 26-2-2012 by CrimpJiggler] |
2-3 milimeters is enough. Hydrogen chloride comes out from the funnel dry, and it encounters water vapor in the beaker and immediately forms a mist
that sinks down.
If you look closely in that few mm space, the funnel behaves as one large cloud, and the mist is falling down like rain. A light source placed
sideways can help you see it better.
Of course, the more concentrated the acid is, the less violent the sucking is and gradually becomes lazy, so after a while, you can lower the funnel
few milimeters into the acid.
But I strongly recommend not submerging it at the begining of the experiment.
Scrubbing - cleaning. Gas scrubbers are devices used to clean the passing gas because they scrub the unwanted matter away.
That yields a very dilute solution of hydrogen chloride; it could be even less saturated than the stomach acid. I've never did the titration of the
ending solution, but something tells me one could even lick it without any problems. 
Both the ammonia and hydrogen chloride fountains can be extremely violent, loud and scary events and even implode larger and less durable glassware.
That's why the whole funnel-in-the-water thing has to be done very carefully. The whole apparatus can shake violently and something might burst or end
up snapped, and glassware is expensive...
|
|
|