cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
Identification of this titanium compound
I was wondering whether anyone had any insight into what this lemon-colored titanium complex could be.
It was prepared by neutralizing purple titanium sulfate solution with sodium bicarbonate until it stopped evolving CO2. At that point, a white
precipitate was recovered by vacuum filtration once the solution was chilled to about 5C.
At that point, an excess of acetyl salicylate was added to the now dry precipitate (titanium carbonate?), and a minimum of distilled water was added
to dissolve the chemicals. With gentle heating, a black precipitate was produced (which looked like powdered titanium metal). This was then chilled
and again vacuum filtered.
What I then noticed was that as the filter paper dried, the material on it started turning yellow, and had the needle like crystals of ASA. The
filtrate was then left to evaporate. Two days later, I returned to the lab to find large yellow crystals that form flat plates.
I'm including a picture.
I can try reproducing this procedure with more careful measurement of the reagents if necessary.
Is it possible that this is titanium acetyl salicylate?
Thanks in advance.

"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
Please post the chemical formulas of all the reactants and go through with a step by step observations. I think it is impossible for it to be titanium
acetyl salicylate. When you added the ASA to the TC and dissolved both tell me (after analysis) what it actually is. But please remember that the
carbonate and the titanium both have to go some ware in this reaction. Please also tell me if CO2 was released.
You can't arrest me, it was for science!
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
What I was thinking was the following:
H2O
Ti(SO4)2 (aq) + 2NaHCO3 (aq) -------------> TiCO3 (s) + 2Na2(SO4) (aq) + CO2 (g) + H2O
When filtered, I was left with the precipitate of TiCO3.
This was washed and reacted with HC9H7O4 (ASA) as follows:
H2O
TiCO3 + 2HC9H7O4 ----------------> Ti(C9H7O4)2 + H2O + CO2
But that doesn't necessarily explain the color change I observed. When copper aspirinate is made in this way, the copper aspirinate immediately falls
out of solution as an extremely unreactive solid. This appeared black, looking like simply reduced titanium powder. When it crystallized, I was
quite shocked. Are these reactions possible? I can't find a lot of information on these titanium compounds other than their empirical formulae.
Also, I haven't done inorganic chem in a while.
Thanks.
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
I can't either. Your equation looks fine. Did so the powder crystallise? this is getting odd. I know asprinates are usually insoluble in water but
soluble in non polar solvents. Take a crystal and add it to isopropenol or ethanol alcohol. If it dissolves it is probable that it is titanium
asprinate. If not in the next post please tell me.
You can't arrest me, it was for science!
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
It did crystallize as the water was evaporated. When it was placed in ethanol, it did dissolve mostly, yielding a pale yellow solution.
I'm attaching pictures of the process.
  
I think perhaps the insoluble component is unreacted sodium bicarb.
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
I would have to agree, I believe we have I identified this as titanium asprinate. Just do one more thing to make sure: Take the ethanol solution and
filter it then recrystallise it you should end up with a clear crystals if this is titanium asprinate and the yellow is other general contaminates.
Sorry for dragging this on so long but it is better to be able to test and prove what you have is what you have than think you have the right compound
when really its not. It seems from these tests that its titanium asprinate 
You can't arrest me, it was for science!
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
If you want my opinion, You cannot have made titanium carbonate since there is no such titanium compound, you probably made hydrated titanium dioxide
and then reacted it with your ASA. A complex is likely to be there with titanium chemistry, I could look in my titanium chemistry book if you want.
I never asked for this.
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
Hmm your correct there is no such compound my guess now is titanium salysilate:
What do you think?
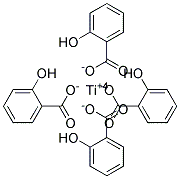
You can't arrest me, it was for science!
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
I allowed the ethanol to evaporate off, and was left with this.

It didn't crystallize. I think plante1999 was right.
Plante: If you could look it up, I would be very much appreciative.
Is it possible that what I observed was actually reduced titanium powder, that the ASA didn't react with the titanium at all, and that the yellow
color is just titanium impurity in ASA crystals?
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
It is probably titanium salicylate, this is what lead salicylate looks like because I could not find titanium salicylate:

I am still open to the idea that it is titanium carbonate! To test for a base take a crystal and pour acetic acid on it. It should react if it is
titanium carbonate.
[Edited on 7-3-2012 by disulfideprotein]
[Edited on 7-3-2012 by disulfideprotein]
You can't arrest me, it was for science!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I remember seeing something on titanium complexed with 2-acetoxybenzoate, back when I was doing my lit. research on dicopper 2-acetyloxybenzoate. I
know I printed it out . . . let me dig through my files to see if I can find the reference.
[edit]
My memory fails me, apparently. I found the papers on Zn and Cd complexes, but not Ti. Sorry.
I have no Ti salts, but I do have some "fused" Ti metal. If I can find the time, I will prepare the sulfate and try to replicate your experiment.
[Edited on 3/8/12 by bfesser]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
First some general remarks. Titanium exists in oxidation states +3 and +4. In oxidation state +3 it forms deep purple or even purplish/blue aqua ions,
which can be written as hydrated Ti(3+). In oxidation state +4 it hardly can exist in aqueous solution as Ti(4+). Titanium(IV) has a VERY strong
tendency to hydrolyse and give hydrated TiO2 in water. Only at very low pH, or in the presence of strongly coordinating agents it remains in solution.
Ti(4+) usually is colorless or white. A well-known exception is the peroxo complex of titanium(IV), which is deep orange/red and even at very low
concentration you still can easily see it as a yellow color.
I have a few questions. What you mention as titanium sulfate, what color has the solid? Is it deep purple, light purple or white? What color has the
solution? Is this solution clear? If the solution is purple then the titanium would be in oxidation state +3.
I understand that after adding bicarbonate you get a white precipitate. This would tell me you have titanium in oxidation state +4. But this cannot be
explained from the starting purple solution. Adding the bicarbonate cannot change the oxidation state from +3 to +4. So, this makes me think that your
titanium sulfate probably is not pure. Is it a commercial sample, or did you make it yourself? Was the precipitate white immediately or did you allow
it to stand for some time and it turned white over time?
If you want clear answers then be very precise about what you did, about the origin of your chemicals (self-made? and if yes, then how were they made
and from what starting materials) and all observations. Right now, there are too many missing pieces of information.
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
If memory serves (my original lab notebook was lost in the move), the starting solution was prepared by dissolving titanium metal (from a titanium
electrode) in 98% sulfuric acid with strong heating. Only a little of the titanium went into solution, and I still have most of the starting metal.
I never attempted to crystallize the "Titanium Sulfate" and just assumed, perhaps incorrectly that that's what it was.
I remember testing a small amount by adding a few drops of it to 30% H2O2 and got the peroxo complex.
I think I'm going to reproduce the procedure one more time, and take photos of each step. Perhaps that will shed some light on what this is (and
maybe what I got wrong).
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
I'm in the process of repeating the experiment.
Something different happened this time when I neutralized the Titanium Sulfate solution. It didn't form a precipitate. It looks like a suspension of
extremely finely divided titanium powder in aqueous solution.
I began by preparing the titanium sulfate solution freshly, by adding titanium metal pieces to hot sulfuric acid, and allowing them to react for 3
hours. I let it cool, removed and rinsed the remaining titanium and then decanted.

I prepared 5 mL of solution and diluted it to 20mL by adding distilled water.

I then added 3.70 g of NaHCO3 until it stopped evolving CO2 gas.
When I first starting neutralizing it, the solution started to become clear,

and at the very end of neutralization, it went almost black.

I vacuum filtered it to remove any remaining insolubles, but wasn't left with very much.
 
I then, suspecting that this is a suspension of titanium powder in sodium sulfate solution, decanted the filtrand into two test tubes to let it
settle.

I will return tomorrow to see what happens and continue the workup.
Edit 4:09pm: Apparently, I was right, and that now it is settling as a flocculant precipitate. I will wait until tomorrow, and until it has fully
settled, and filter again with finer pore paper.
 
[Edited on 3-9-2012 by cAMP]
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
cAMP
Harmless

Posts: 24
Registered: 6-7-2011
Location: East Coast, USA
Member Is Offline
Mood: No Mood
|
|
The next morning, I entered the lab to find something rather surprising. The precipitate that was accumulating at the bottom of the test tubes had
consolidated, floated to the top, and was turning white.

I filtered both test tubes by gravity filtration,

and recovered a wet product.

Since it appears that this is oxygen sensitive, I placed it in my makeshift dessicator

to dry it as quickly as possible. I will update when I have recovered a dry product and can continue the procedure.
"You just spent the entire night arguing grand unification theories with Albert Einstein!" - Geordi LaForge
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cAMP  |
Something different happened this time when I neutralized the Titanium Sulfate solution. It didn't form a precipitate. It looks like a suspension of
extremely finely divided titanium powder...
|
If your titanium did react the first time to form the sulfate, I can't imagine how you could reduce it to the metal. Most metals are not possible to
get back from salts easily once formed. That would require a reduction, which would either be chemical or electrical. I would think that either
you had titanium metal left in the "sulfate" or that you simple formed a titanium oxide/hydroxide hydrate as was previously suggested. Many of those
are white/grey/or dark solids, and the oxidation and hydration states can change with time, water, pH, and oxidation. EG, Aluminum forms many
oxides, hydroxides, and hydrates depending on the situation.
I could see the Ti forming a trivalent salt with the salicylicate, but I don't know much about those salts. But most Ti reactions I have done ended
up with Ti oxides as a white ppt. It makes great paint...
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by cAMP  | The next morning, I entered the lab to find something rather surprising. The precipitate that was accumulating at the bottom of the test tubes had
consolidated, floated to the top, and was turning white.
I filtered both test tubes by gravity filtration,
and recovered a wet product.
Since it appears that this is oxygen sensitive, I placed it in my makeshift dessicator
to dry it as quickly as possible. I will update when I have recovered a dry product and can continue the procedure.
|
You made titanium III hydroxide a black solid which disproportionated/was oxidized by H2O to hydrous titanium dioxide, Ti(OH)2O which is white, After
a few hours the hydrous titanium dioxide will be titanium dioxide nonahydrate.
Hop it helped!
I never asked for this.
|
|
|