qdung92ct
Harmless

Posts: 10
Registered: 12-1-2012
Member Is Offline
Mood: No Mood
|
|
Crystallization
Hi
I'm going to do a gift for my friend and I got an idea
I wanna make something that is formed from the recrystallization of solutions (may be Copper II Sulfate or the Zinc ionic)
So can I control the recrystallization with form that I want ?
For example, the crystal flower
Thanks
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Possibly; look into using different containers and cooling patterns.
Cooling a hot solution quickly produces very small crystals, yet cooling very slowly, maybe even by putting the beaker in a thermos, will produce
larger ones.
The best crystals, of course, will come from allowing a RT solution to crystallize very slowly on its own.
Read up a bit on it, there are plenty of YouTube videos and instructions explaining questions just like this.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Growing crystals that are worthy of a gift is a business of patience. In essence the slower you grow them, the more 'perfect' they tend to be. Also:
purer materials tend to give more perfect crystals, so a quick recrystallisation of your starting material can pay off handsomely later on.
Popular crystal growing materials, often found in commercial crystal growing kits, are alum (potassium aluminium sulphate dodecahydrate) and ammonium
dihydrogenphosphate, both of which are fairly OTC. There are clear (white) but can be coloured with food dyes for special effects: green alum looks a
bit like emeralds, for instance.
As Hexavalent said: search the web and Utoob for manuals.
[Edited on 17-3-2012 by blogfast25]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Not exactly crystal growing but..........
A great way to create impressive looking art pieces is to 'grow' metal 'trees' from solutions of the metal salts using electricity.
I did this years ago using Copper Sulphate and some Copper wire as the Anode and the Cathode (+ & -).
You can get exquisit looking Copper trees growing from the wires if you use some diodes and resistors as per the diagram (as far as I can remember).
The trees care extreamly dendritic and complex and will look very pretty if left in the original growing solution.
Dann2
[Edited on 17-3-2012 by dann2]
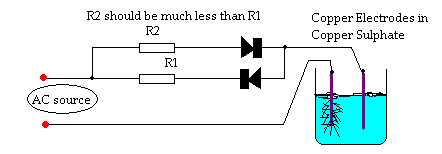
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Not a DC current source dann2?
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
MrTechGuy1995
Harmless

Posts: 27
Registered: 21-9-2011
Location: Chatham, New Jersey
Member Is Offline
Mood: Lab Envy
|
|
It converts to DC through AC.
One line is Live, and the other is "Ground".
The symbol that looks like a "Play next to a box" Is a diode. Put 2 of them together and you get a Bridge Rectifier.
Basically a Single Direction Current that has a wave of:

I can't believe I spent all freshman year studying this during my lunch hour. But it was well worth it!
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Thank you Mrtechguy! Electricity is definitely not my strong suit. It time I started to read up
[Edited on 17-3-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Please note that many "large" crystals may look beautiful but they often have a fatal flaw...
they are brittle as hell and break apart quite easily.
I tried with Manganese Chloride and Copper Sulfate and even after a good drying, they tend to fracture very easily so don't think you'll make
something as hard as a Topaz or Quartz crystal cluster unfortunately.
Maybe there are other chemicals that produce more sturdy crystals... plain old NaCl might be the trick.
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dann2  | ...
You can get exquisit looking Copper trees growing from the wires if you use some diodes and resistors as per the diagram (as far as I can
remember).[Edited on 17-3-2012 by dann2] |
Your circuit will produce an AC current, though with diode-drop votage offsets near zero crossing. Full-wave rectification with two diodes is
accomplished with a centre-tapped transformer; lacking that you need four diodes connected in a bridge configuration.
To get the pulsed-DC waveform you show, simply remove one of the diodes.
-Boby
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
The silver tree is a classic demonstration of metal growing:
http://www.unitednuclear.com/silvertree.htm
Maybe if drained and washed with alcohol it could be backfilled with acrylic and preserved.
Another interesting metal 'tree' that I grew involved tin rods. I had large bars of tin used for solder and I tried to dissolve a whole bar in
hydrochloric acid. Over the days it dissolved and then strangely started to plate back out on the bar in the form of needles, less at the top, more
at the bottom until it did start to resemble a tree. It was very pretty, turns out the tin had 2% silver in it to help it flow. I am assuming this
was again silver crystals.
As Hexavalent stated above, allowing a solution to grow crystals slowly is a must for nice well formed crystals. It took the better part of 8 months
for a large (2L) solution of copper sulfate to evaporate entirely at room temp but I ended up with some crystals that would look nice in jewelery and
could be cut and polished if desired.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
The circuit I give above was from a dry-cell-battery-rejuvenator circuit.
I was messing around with it years ago and (cannot remember quite why) then started to mess around with solutions of Copper Sulphate and Copper
electrodes.
The circuit gives a small amount of current reversal (depending on size of resistors) during each cycle (visualize small humps under the line in
MyTechGuy1995 diagram). This seemed (I thought) to give more dendritic 'trees' than a straight DC. Perhaps DC is just as good at giving lots of
dendrites/branches. Using one diode in my circuit will give you DC, or use a bridge retifier or just a plain old purchased DC power supply.
Crystals and Copper trees or Silver trees always seem to look better when in solution. When you take them out they often loose some of their beauty.
DANN2
D
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
BromicAcid: I saw a journal article the other day mentioning the recrystallization of tin from hydrochloric acid solutions but I cannot for the life
of me re-find the said article. It mentioned that alloyed tin worked better, and that it was a result of some equilibrium between the tin metal and
the tin (II) chloride produced upon the action of acid.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I've done this demo a few times, and the tree is extremely fragile. The crystals are just so tiny and they don't adhere very well to the copper wire,
so they tend to fall off at the slightest bump. I agree it would be very beautiful encased in acrylic, I just don't think the tree would survive the
process.
|
|
|