Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Cyclicization of Acetylene
| Quote: |
If dicarbonylbis(triphenylphosphine)nickel
[13007-90-4], Ni(CO)2[(C6H5)3P]2 , is used as
catalyst, the cyclization products are benzene
(88% yield) and styrene (12% yield). The reaction
is carried out in benzene at 65– 75 ◦C
and 1.5MP .
Ullmann's Enc. of Industrial Chemistry
|
Somebody has more information about this procedure?
I have Roth autoclave and I like to test this method.
Also i found below paper
Attachment: acetylene _to_benzene.pdf (354kB)
This file has been downloaded 710 times
[Edited on 3-4-2012 by Waffles SS]
|
|
|
Aperturescience27
Harmless

Posts: 39
Registered: 5-4-2012
Member Is Offline
Mood: No Mood
|
|
I found this http://en.wikipedia.org/wiki/Walter_Reppe#Reppe_chemistry (look at the bottom of the "Reppe Chemistry" section) It mentions triphenylphosphine and
nickel.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
There's some related articles uploaded at:
https://the-collective.ws/forum/index.php?topic=12601
the reported yield of benzene is about 80% 
Chemistry is our Covalent Bond
|
|
|
madcedar
Hazard to Others
  
Posts: 116
Registered: 10-9-2009
Member Is Offline
Mood: No Mood
|
|
Got an account and waited for access but I get this message when I click on the link in the above post.
"The topic or board you are looking for appears to be either missing or off limits to you."
So I guess your access to The Collective is at a higher level than someone who has just signed up.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Got an account and waited for access but I get this message when I click on the link in the above post. "The topic or board you are looking for
appears to be either missing or off limits to you." |
One is required to participate a little at the Collective to gain access to most of the content  You can always do your own library research if that's too much to ask You can always do your own library research if that's too much to ask 
Chemistry is our Covalent Bond
|
|
|
madcedar
Hazard to Others
  
Posts: 116
Registered: 10-9-2009
Member Is Offline
Mood: No Mood
|
|
 Library Research he he Library Research he he 
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
The main problem with making benzene from acetylene is the temperature/pressure what it goes on.
I have red a book (written in 1940) where this method was described and the producere was the following: pass some acetylene gas into a steel tube
filled with iron flakes heated until it's red hot. Benzene should come over in a relative moderate yield.
This method have been improved several times, the usage of the metal-organic catalysts improved it a lot, the only problem is the price of the MO
catalysts (and the preparation of them if You want want to make it home)... It's not that easy.
And also in the document, mentioned by Waffles-guy there is a thing: pressure. 1.5MP is really-really much. It could hurt.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
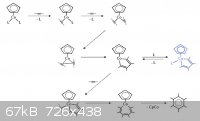
One of my friend told me cyclopentadienylcobalt dicarbonyl ([CpCo(CO)2]) has better yield than triphenylphosphinonickel
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  | | One of my friend told me cyclopentadienylcobalt dicarbonyl ([CpCo(CO)2]) has better yield than triphenylphosphinonickel |
That's true.
The problem here is also with the catalyst, it's hard to prepare it and also a short note: cyclopentadiene is a bad stuff. It dimerizes into
dicyclopentadiene what should be pyrolised to give cyclopentadiene and this pyrolisis is a really "smelly" and dirty reaction even with relative small
amounts.
And CpCo(CO)2 is sensitive to air, moisture and highly toxic.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
IMHO I don't see how this synthesis is interesting for an amateur other than from a purely academic perspective - i.e. just for the heck of it. As the
decarboxylation of benzoates is well documented, cheap, OTC and incredibly easy to perform I can't see the appeal of this route if the goal is to
prepare useful quantities of benzene. Also, I believe this topic (benzene from acetylene) was discussed in the earlier benzene thread (UTFSE), but I
don't remember if anything valuable came out of it.
However, something that is interesting, is that cyclooctatetraene can be prepared from acetylene with much more amateur-friendly catalysts. To quote myself from Magpie's autoclave thread:
Quote: Originally posted by Lambda-Eyde  |
Have you performed any other high-pressure syntheses with the autoclave, or do you have any plans? While reading about sandwich compounds
(metallocenes etc.) I noticed some complexes with cyclooctatetraene (COT). COT is by no means cheap, my local supplier sells 1g at ~50$. The cheapest
I can get is 250 mg for half the price. I was surprised to find out that COT can relatively easily be synthesized by heating acetylene with nickel
cyanide and calcium carbide in THF at 15 atm/60 degrees C - well below the maximum operating conditions of your autoclave. And it gives 90 % yield!

The original reference by Reppe (in German) can be found here.
|
COT is quite an interesting molecule - it can be used to prepare different organometallic complexes, one example is uranocene - now that's a conversation starter!  It also forms
explosive peroxides upon standing, much like diethyl ether. Also, COT is horribly expensive (at least in my neck of the woods), so if anyone were to
prepare some I'd be willing to buy a few grams (of course for a symbolic price It also forms
explosive peroxides upon standing, much like diethyl ether. Also, COT is horribly expensive (at least in my neck of the woods), so if anyone were to
prepare some I'd be willing to buy a few grams (of course for a symbolic price  )
to experiment with. )
to experiment with.
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I doubt that acetylene can survive such high pressures. I heard it explodes when compressed.
Rest In Pieces!
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by Adas  | | I doubt that acetylene can survive such high pressures. I heard it explodes when compressed. |
Yes, that's why the acetylene you buy in tanks is actually a solution in acetone (under pressure). But the above replies aren't speculations, they're
documented, industrial procedures - so someone has obviously done it earlier without blowing themselves up. If anyone has information on this (IMO)
odd behaviour of acetylene, I think all of us would be interested to read about it.
Edit: Wikipedia has this to say:
| Quote: | | For use in welding and cutting, the working pressures must be controlled by a regulator, since above 15 psi acetylene will oligomerize explosively.
|
Which only makes me more confused, as 15 psi really isn't that much!
[Edited on 20-5-2012 by Lambda-Eyde]
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by kristofvagyok  | The main problem with making benzene from acetylene is the temperature/pressure what it goes on.
I have red a book (written in 1940) where this method was described and the producere was the following: pass some acetylene gas into a steel tube
filled with iron flakes heated until it's red hot. Benzene should come over in a relative moderate yield.
|
Can you put your reference.It seems this method doesnt require Cobalt or Nickel catalyst
| Quote: |
I doubt that acetylene can survive such high pressures. I heard it explodes when compressed.
|
I can buy acetylene capsule here that has 15Bar pressure
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
compressed (non-adsorbed) acetylene is more liable to explode than non-compressed, if concussed. It doesn't just spontaneously go on it's own. But
who knows, on a bumpy transport? As one poster alluded, that's why the tanks contain a spongy matrix saturated w/ DMK, which absorbs the acetylene.
Just as if, you were to wet an intimate mixture of KCLO3 + confectioners sugar, it wouldn't go when hit with a hammer on a boulder, as if it were dry.
Same theory I think. Maybe I'm off.
|
|
|