Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Favorskii rearrangement question
Has anyone tried this reaction on aromatic chloromethyl ketone? I am thinking of doing a Friedel Crafts acylation on 1,4-dimethoxybenzene with
chloroacetyl chloride, AlCl3, followed by Favorskii rearrangement with say sodium ethoxide to give ethyl-1,4-dimethoxyphenylacetate. Most of the
literature that I have seen using Favorskii rearrangement seems to be with aliphatic substrates.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You must be confused in regard to the Favorskii rearrangement, because an aryl chloromethyl ketone obviously can not undergo such a rearangement: you
don't have any C-H groups where it should be. See the mechanism of the rearrangement to understand more.
Perhaps you believe your substrate can undergo a different rearrangement? The only rearrangements I can think of that give arylacetic acids from aryl
chloromethyl ketones are: a) the rearrangements that occur during their nucleophilic substitution via neighboring group participation; b)
Benzylic-like rearrangements.
The rearrangements of the first type are well documented and mechanistically studied. I don't remember if I ever saw them on phenacyl chlorides, but
they should proceed at least on 4-hydroxyphenacyl chlorides. I know of 2-(4-hydroxyphenyl)ethyl halides that undergo this type of rearrangements
easily, but the non-hydroxy substituted phenethyl halides generally require other promoting conditions to be meet (like in the case of the textbook
Ph3CCH2Cl rearrangement of this kind). You should do a literature search at least, but in any case your specific substrate does not look suitable at
all.
I have a vague recollection that the rearrangements of the second type were already discussed on this or some other forum. Organikum posted some
Chinese reference about it. Phenacyl chlorides are not very suitable substrates, so the selectivity and yields are expected to be low.
PS: Please open referenceless threads in the Beginnings section only. See the Guidelines for posting on the ScienceMadness forum for more info. Once you equip this topic with some starting point references, someone might be
tempted to do a thorough literature search.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
11-4-2012 at 07:29 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
It sounds as if the OP is looking for a route to (esters of) phenylacetic acids. The corresponding acetophenones can be converted using the Willgerodt
reaction, using morpholine and sulfur. Base hydrolysis of the resulting thioamide produces (after acidification) the phenylacetic acid.
|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Thanks Nicodem for reminding me of this mechanism. I must have been up too late when I was thinking about this, you are correct, an alpha H is
required for deprotonation. I ran out of this starting material so was looking for ways to make it again. The next steps are pretty obvious, thionyl
chloride, ammonia, LAH reduction ....etc.
|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
See this scheme from Italian paten. Maybe this is a B.S. patent but they do give a yield, or perhaps I don't understand all of the details in second
reaction that they are calling Favorskii rearrangement.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Which patent? You don't give the reference!
| Quote: | | Maybe this is a B.S. patent but they do give a yield, or perhaps I don't understand all of the details in second reaction that they are calling
Favorskii rearrangement. |
The reaction on the scheme looks totally viable, but it is definitely not a Favorskii rearrangement. It is the type of rearrangement I discussed in my
previous reply under the description (a). The acetal intermediary is to block any other pathway, apart from the desired rearrangement. Quite an
ingenious solution to the problem, I might say, but it adds two more reaction steps. In this view, the suggestion by DJF90 is much more practical, if
all you want is 2,5-dimethoxyphenylacetic acid rather than being interested in the rearrangements and their chemistry.
|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Sorry I did not include references. Here are two references with more detailed explanation in Bayer patent on the dimethyl analog. Anyway I don't read
Italian but maybe they have other patents in English.
Process for the preparation of 2,5-dihydroxyphenylacetic acid from 1,4-dimethoxybenzene via Friedel-Crafts acylation and Favorskii rearrangement.
Banfi, Stefano; Quici, Silvio. (Recordati Industria Chimica e Farma Ceutica SpA, Italy). Ital. Appl. (1992), 16pp. CODEN: ITXXCZ IT
91MI1310 A1 19921115 Patent written in Italian. Application: IT 1991-MI1310 19910514. Priority: IT 1991-MI1310 19910514. CAN 155:380058
AN 2011:1164942 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
IT 91MI1310 A1 19921115 IT 1991-MI1310 19910514
Priority Application
IT 1991-MI1310 19910514
Production of 2,5-dimethylphenylacetic acid. Himmler, Thomas. (Bayer Cropscience Aktiengesellschaft, Germany). PCT Int. Appl. (2005), 26
pp. CODEN: PIXXD2 WO 2005075401 A1 20050818 Designated States W: AE, AG, AL, AM, AT, AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU,
CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, HR, HU, ID, IL, IN, IS, JP, KE, KG, KP, KR, KZ, LC, LK, LR, LS, LT, LU, LV, MA, MD, MG,
MK, MN, MW, MX, MZ, NA, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RU, SC, SD, SE, SG, SK, SL, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, YU, ZA,
ZM, ZW. Designated States RW: AT, BE, CH, CY, DE, DK, ES, FI, FR, GB, GR, IE, IS, IT, LU, MC, NL, PT, SE, TR, BF, BJ, CF, CG, CI, CM, GA, ML, MR, NE,
SN, TD, TG. Patent written in German. Application: WO 2005-EP617 20050122. Priority: DE 2004-102004005318 20040204; WO 2005-EP617
20050122; US 2006-586491 20060720. CAN 143:231764 AN 2005:811725 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
WO 2005075401 A1 20050818 WO 2005-EP617 20050122
W: AE, AG, AL, AM, AT, AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, HR,
HU, ID, IL, IN, IS, JP, KE, KG, KP, KR, KZ, LC, LK, LR, LS, LT, LU, LV, MA, MD, MG, MK, MN, MW, MX, MZ, NA, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RU,
SC, SD, SE, SG, SK, SL, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, YU, ZA, ZM, ZW
RW: BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, ZW, AM, AZ, BY, KG, KZ, MD, RU, TJ, TM, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR,
GB, GR, HU, IE, IS, IT, LT, LU, MC, NL, PL, PT, RO, SE, SI, SK, TR, BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, ML, MR, NE, SN, TD, TG
DE 102004005318 A1 20050825 DE 2004-102004005318 20040204
EP 1713755 A1 20061025 EP 2005-701122 20050122
EP 1713755 B1 20100113
R: AT, BE, CH, DE, DK, ES, FR, GB, GR, IT, LI, LU, NL, SE, MC, PT, IE, SI, LT, FI, RO, CY, TR, BG, CZ, EE, HU, PL, SK, IS
CN 1918103 A 20070221 CN 2005-80004129 20050122
BR 2005007422 A 20070626 BR 2005-7422 20050122
JP 2007522139 T 20070809 JP 2006-551756 20050122
JP 4668212 B2 20110413
AT 455087 T 20100115 AT 2005-701122 20050122
IN 2006DN04004 A 20070824 IN 2006-DN4004 20060712
US 20080234501 A1 20080925 US 2006-586491 20060720
US 7579500 B2 20090825
US 20090156839 A1 20090618 US 2009-388274 20090218
US 7629476 B2 20091208
Priority Application
DE 2004-102004005318 A 20040204
WO 2005-EP617 W 20050122
US 2006-586491 A3 20060720
Abstract
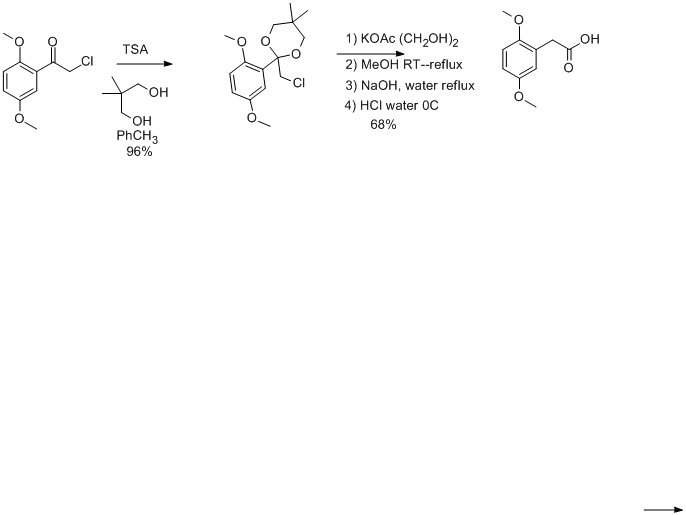
The invention relates to a method for prodn. of 2,5-dimethylphenylacetic acid by reacting p-xylene with chloroacetyl chloride in a Friedel-Crafts
reaction to form 2-chloro-1-(2,5-dimethylphenyl)ethanone, which reacts with a diol of the formula HO-CH2-X-CH2-OH to form a spiroketal of the formula
(I), the radical X being CH2, CHCH3, CEt2, or CMe2. The spiroketal is then rearranged into a mixt. of compds. (II) and (III), which is then sapond.
to form 2,5-dimethylphenylacetic acid, the radical X being the same as above. Thus, aluminum chloride (293,2 g, 2.2 mol) was added into a mixt. of
p-xylene (800 g, 2 mol) and chloroacetyl chloride (226 g, 2 mol) over 75 min at 12-15, the reaction mixt. was stirred for 2 h at
12-15, and for 30 min at room temp., ice-water (3,000 mL) and concd. HCl (70 g) were added, and 2-chloro-1-(2,5-dimethylphenyl)ethanone (96.8%
yield) was obtained after a std. work up procedure. The above ketone (0.5) reacted with 2,2-dimethyl-1,3-propanediol (1) in the presence of p-TsOH
hydrate (0.05 mol) to afford the resp. spiroketal (94.6% yield), which was rearranged in ethylene glycol at 180-185 in the presence of sodium
acetate, and sapond. with 30%-aq. NaOH at 100-105 to obtain 2,5-dimethylphenylacetic acid (99.3% pure, 93.7% yield).
Compound II is the dimethylphenylacetic acid ester of whatever diol used in protecting the ketone, III is the diol esterified on both sides with
dimethyl phenylacetate.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | In this view, the suggestion by DJF90 is much more practical, if all you want is 2,5-dimethoxyphenylacetic acid rather than being interested in the
rearrangements and their chemistry. |
Even more so that the original poster seems to be interested in the amide.
An interesting route to phenethylamines.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Qadira  | Anyway I don't read Italian but maybe they have other patents in English.
Process for the preparation of 2,5-dihydroxyphenylacetic acid from 1,4-dimethoxybenzene via Friedel-Crafts acylation and Favorskii rearrangement.
Banfi, Stefano; Quici, Silvio. (Recordati Industria Chimica e Farma Ceutica SpA, Italy). Ital. Appl. (1992), 16pp. CODEN: ITXXCZ IT
91MI1310 A1 19921115 Patent written in Italian. Application: IT 1991-MI1310 19910514. Priority: IT 1991-MI1310 19910514. CAN 155:380058
AN 2011:1164942 CAPLUS |
Well, I understand Italian. The patent application apparently changed its priority number after it become a patent and is now IT1247923 (searching by
the number given in your CA leads to nowhwere). The inventors apparently have troubles with name reactions: Not only did they misname the title
rerrangement, but in the discussion they also misname an aromatic Claisen rearrangement for a Fries rearrangement. This is irrelevant in regard to
their process validity, though. You might be interested in that they thoroughly reviewed the literature in regard to the homogentisic acid syntheses,
especially those routes that go trough the 2,5-dimethoxyphenylacetic acid as the final intermediate. For the idea of the acetal rearrangement they
point to Synthesis 1985, 436 and Synthesis 1985, 505. The rearrangement conditions are 3.5 h of heating at ethylene glycol reflux
with potassium acetate as the base. This supposedly gives a mixture of esters (orthoesters should be the product of such a reaction in my opinion, but
perhaps they decompose to esters at such harsh conditions). This mixture of esters is not isolated, but a solvent exchange is done with methanol and
then they are hydrolyzed to the carboxylate in with KOH(aq)/MeOH.
I did not check the Synthesis articles or the other patent, but from what I know and from what I read, it looks like this type of
rearrangement works fine.
The Friedel-Crafts acylation with chloroacetyl chloride might not be as simple as it sounds in that patent or elsewhere. One member at the Hyperlab
forum posted his experience with this reaction that you should read before you do anything. As far as I remember, he could not make it work in
dichloromethane at all, and only performing the reaction in nitromethane as the solvent worked for him (or maybe it was a CH3NO2/CH2Cl2 mixture, don't
really remember anymore). Personally, I find this a bit strange as acylations with other acyl chlorides generally work at the same conditions (apart
the losses form demethylation), so perhaps his chloroacetyl chloride or some other reagent was compromised.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Some details on starting material prep by Friedel Craft. It seems slow addition of Chloroacetylchloride is important.
Italian patent claims 99% yield with AlCl3, DCE, for AlCl3 addition rt -> -5C, looks like 30min addition of acid chloride at -5C, then 2 hr -5C’
-5 --> RT 15h, usual workup on crushed ice HCl.
Synthesis of 2-Bromo-1-(2,5-dimethoxyphenyl)ethanone. Zhang, Jun-jie; Li, Hong-xia. College of Chemical Engineering and Biotechnology, Hebei
Polytechnic University, Tangshan Hebei, Peop. Rep. China. Hebei Ligong Xueyuan Xuebao (2007), 29(1), 95-96,101. Publisher: Hebei Ligong
Xueyuan Xuebao Bianjibu, CODEN: HLXUFU ISSN: 1007-2829. Journal written in Chinese.
2nd Best yield reported 96% CCl4, AlCl3, cooled; 1.5h cooled, 5h RT
Synthesis of midodrine hydrochloride by the reduction of the novel intermediate 1-(2',5'-dimethoxyphenyl)-2-azidoethanone. Ray, Anup K.; Patel,
Hiren; Patel, Mahendra R. (Geneva Pharmaceuticals Inc., USA). U.S. (2001), 5 pp. CODEN: USXXAM US 6201153 B1 20010313 Patent written
in English. Application: US 2000-550417 20000417. Priority: US 2000-550417 20000417; WO 2001-EP4348 20010417.
64% yield AlCl3, DCM. 60g DMB; AlCl3 added, then over 1.5h add acid chloride dropwise at RT, stir 4h then workup. This patent can be downloaded from
google patents. They also got same yield from refluxing chloroacetic anhydride.
Another Synthesis paper describes the chlorination of the acetophenone with exotic chlorinating agent benzyltrimethylammonium dichloroiodate with 95%
yield. Ref:
Halogenation using quaternary ammonium polyhalides. Part X. .alpha.-Chlorination of aromatic acetyl derivatives with benzyltrimethylammonium
dichloroiodate. Kajigaeshi, Shoji; Kakinami, Takaaki; Moriwaki, Masayuki; Fujisaki, Shizuo; Maeno, Kimihiro; Okamoto, Tsuyoshi. Fac. Eng.,
Yamaguchi Univ., Ube, Japan. Synthesis (1988), (7), 545-6.
Synthesis of 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide (midodrine hydrochloride). Hu, Honggang; Zhang, Jun; Zhao, Qingjie; Zhao,
Huiqing; Wu, Qiuye. School of Pharmacy, Second Military Medical University, Shanghai, Peop. Rep. China. Zhongguo Yiyao Gongye Zazhi (2006),
37(7), 445-446.
Again Friedel Crafts 78% yield; AlCl3, CS2 as solvent 0C 12h
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
I still don't understand why you would do this arcane transformation via the phenylacetic acid, when according to:
you are interested in the amide? A Willgerodt rearrangement of the acetophenone would directly give the amide. Is there any reason this wouldn't work?
*confused*
|
|
|
Qadira
Harmless

Posts: 7
Registered: 10-4-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | I still don't understand why you would do this arcane transformation via the phenylacetic acid, when according to:
you are interested in the amide? A Willgerodt rearrangement of the acetophenone would directly give the amide. Is there any reason this wouldn't work?
*confused* |
At this time I don't have any sulfur in large quantities. Willgerodt works best with morpholine which I do have, however I don't want to make the
thioamide of morpholine even though I could hydrolyze it to the acid. I am working with reagents that are available to me at this time. I have a fully
equipped lab though with fume hoods to work safely, and access to LC mass spec, nmr etc for analysis or even preparative chromatography in normal or
reverse phase. So I can guarantee you I won't be taking melting points 
|
|
|
|