| Pages:
1
..
4
5
6
7
8
..
23 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Who knew that a seeming inert ceramic , Boron Nitride , can serve as fuel
in a sprengel admixture. This is literally OTC but details are sparingly available.
It appears that BN powder soaked with fuming nitric acid is comparable to
some blends of aromatics with tetranitromethane.
The Future of Warheads Armor & Ballistics
mentiond on page 7
http://www.mater.upm.es/ISB2007/Proceedings/PDF/Volume_1/Vol.I(1)GS01.pdf
Reaction Mechanisms in Shocked, Intercalated Graphite and Boron Nitride
http://hal.archives-ouvertes.fr/docs/00/25/37/51/PDF/ajp-jp4...
related thread _
http://www.sciencemadness.org/talk/viewthread.php?tid=13189
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Regarding the Silicon explosive compound disclosed here _
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
Another investigation of it.
Explanation of the Colossal Detonation Sensitivity of Silicon Pentaerythritol Tetranitrate (Si-PETN) Explosive
http://www.wag.caltech.edu/publications/sup/pdf/806.pdf
A related thread _
http://www.sciencemadness.org/talk/viewthread.php?tid=1244#p...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Ramiel had cited this some time back
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
Syntheses of 1,2,3,4-Tetrazine Di-N-oxides, Pentazole Derivatives, Pentazine Poly-N-oxides, and Nitroacetylenes
http://handle.dtic.mil/100.2/ADA445136
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA445136&Locati...
Synthesis of 1,2,3,4-Tetrazines, 1,2,3,4-Tetrazine Di-N-oxides, Pentazole Derivatives, Pentazine Poly-N-oxides, and Nitroacetylenes
http://handle.dtic.mil/100.2/ADA430332
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA430332&Locati...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
No explosive properties discussed but related to this previous post
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
High Temperature Oxidation of Boron Nitride
http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/2001006...
Intercalation of Hexagonal Boron Nitride by Strong Oxidizers
http://www.physics.berkeley.edu/research/zettl/pdf/234.SolSt...
.
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nothing but Nitrogen
Novel High Pressure Structures of Polymeric Nitrogen
http://mysbfiles.stonybrook.edu/~aoganov/files/Nitrogen-PRL-...
Novel High Energy Density Materials Synthesis by Megabar Hot Pressing
http://www.osti.gov/bridge/servlets/purl/231384-GWqCC5/webvi...
Attachment: Polymeric Nitrogen.pdf (673kB)
This file has been downloaded 1698 times
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Has this been postet already:
http://teroras.sprogmenys.net/kestonei/Nitroureas%20II.%20Sy...
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Does someone have this pdf?
http://onlinelibrary.wiley.com/doi/10.1002/prep.19940190504/...
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
Have been working on a new site for lesser known energetic compounds:
https://sites.google.com/site/energeticchemical/home
or for the site directory:
https://sites.google.com/site/energeticchemical/system/app/p...
MonoMethyl Hydrazinium Nitroformate has a det. velocity of 9.134km/sec. This compares with 8.93 for RDX. MMHNF is also 212% more powerful than TNT on
a weight basis, compared with 163% for RDX. The two compounds have similar sensitivity. The article claims, "The decrease in impact sensitivity on
alkyl substitution can be explained on the basis of the increase in basicity of hydrazine on alkyl substitution, entailing stronger holding of the
nitroformate group, leading to greater stability." However, I am uncertain if this is correct. I would think adding methyl groups would decrease
basicity, as holds true for amines (with the exception of the tetramethyl ammonium anion that serves as a superbase). A more likely reason would be
that the methyl groups are more inert to combustion, and so dilute the more sensitive hydrazine, while at the same time improving the oxygen balance.
Journal of Chem. Tech. Vol12 2005
"Synth, Characterization, and thermal behaviour of hydrazinium nitroformate..."
H.S. Jadhav, M.B. Talawar (India)
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Someone got more about this.
Please!
http://resources.metapress.com/pdf-preview.axd?code=h623qq36...
|
|
|
AH-Poster
Harmless

Posts: 9
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
Dinitro-[di-Furazanyl-Hydrazine]
This is an experimental compound that very few people know about. There was a very short synthesis on the "Controversial Chemlab",
http://web.mit.edu/semenko/Public/Military%20Manuals/RogueSc...
but now here is a more substancial synthesis. I do not know how powerful this compound is, but Azidonitroazoxyfurazan, which is the same thing exept
with a --N=N-- in the center instead of --NHNH--, is stated as having 120% the power of HMX in a patent.
This compound has a structure of (NO2)(C2N2O)NHNH(C2N2O)(NO2), where (C2N2O) is a furazan ring. It is also known as dinitrohydrazofurazan. This
compound probably has a power between RDX and HMX, with a lower sensitivity. The gas products from decomposition would be hoter than that from RDX,
but the compound is probably less energetic than the nitramines. The linking hydrazine group may make the compound less chemically and thermally
stable than the nitramines, although since the hydrazine is electron donating, it will be less susceptable to oxidation than otherwise. The furazan
rings do not lend themselves to aromaticity, so the extent of the hydrazine stabilizing the molecule through electron donation will be severely
curtailed. The presence of the hydrazine will allow molecules to pack closer together through hydrogen bonding, the hydrazine also serving to link
together two nitrofurazan groups, giving a higher density than the nitramines.
Synthesis:
To 25mL ethanol in a 100mL round bottomed flask, 0.013g (0.00022 moles) glacial acetic acid was added. To this was added 0.05g (0.0002 moles)
DinitroAzoFuroxan, and 0.5g iron wire (Zn powder may substitute). This is stirred at reluxed for one hour, then 25mL water and about 1g sodium
bicarbonate was added. This was extracted with three 20mL allotments of methylene chloride CH2Cl2. The extracts were washed twice with water and the
resulting solution was then dried with anhydrous MgSO4 and filtered. The solvent was removed to leave yellow crystals, which had a melting point over
a a 160-171degC transition. The compound exothermically decomposes at 215C. If heated rapidly 12deg beyond this, it detonates.
DinitroAzoFurozan Precursor:
In a 250mL round bottomed flask, 0.40g AminoNitroFurazan was dissolved in 16mL concentrated HCl (0.53mol). 0.95g (0.006mol) Potassium permanganate was
dissolved in 105mL water. The acid solution in the round bottomed flask was heated to 40degC, then the permanganate solution was slowly dripped into
the round botomed flask, over the period of 1 hour. The resulting dark brown solution was heated to a little under 57degC for 2 hours with continued
stirring. The solution was cooled, then it was extracted with four 50mL allotments of methylene chloride. The extracts were washed with water, then
dried with anhydrous MgSO4 and filtered. The remaining solution was evaporated under reduced pressure until an orange oily liquid remained, which
contains Diamino di-Furazanyl-Hydrazine; to crystallize the compound out, it must be repeatedly dried with organic solvent and evaporated, and finally
be chilled.
AminoNitroFurazan
100mL flask placed in ice bath and constantly stirred, 56mL 50% H2O2 solution (0.97mol) was added, keeping temperature under 5degC. 5g of sodium
tungstate Na2WO4 was added, 1.1g of DiaminoFurazan was added and allowed to react for 12 hours. The resulting yellow solution was neutralized with
sodium carbonate until neutral pH was reached. The solution was filtered and extracted, similar to the procedures described above. Yellow Crystals
were obtained on evaporation, melting over 120-125degC, with explosion at 170degC.
These are tested procedures, however a theoretical shortcut would be to bubble a limited quantity of NO2 into the furazan of ethylene, giving
mono-nitrofurazan, which has a significantly lower vapor pressure than the original reactant, then addition of chlorine will make nitro,chloro
furoxan. This will condense with symetric diacetyl hydrazine, and this will slowly hydrolyze in hydrazine hydrate to form
Dinitro-[di-Furazanyl-Hydrazine]. Diacetyl hydrazine can be made by treating concentrated N2H4 with Ac2O, no unsymetric diacetyl hydrazine will be
produced. Note that both nitrogen dioxide and chlorine react at room temperature with the furazan of ethylene, since double bonds exist on the carbon.
[Edited on 17-10-2010 by AH-Poster]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AH-Poster  | Dinitro-[di-Furazanyl-Hydrazine]
" I do not know how powerful this compound is, but Azidonitroazoxyfurazan, which is the
same thing exept with a --N=N-- in the center instead of --NHNH--, is stated as having
120% the power of HMX in a patent." / / " also known as dinitrohydrazofurazan "
|
Given the comparable density of ~ 1.8 , my understanding is that performance can be
expected to closely coincide with that of PETN.
A depiction of the structure goes a long way to obviating confusion arising from naming
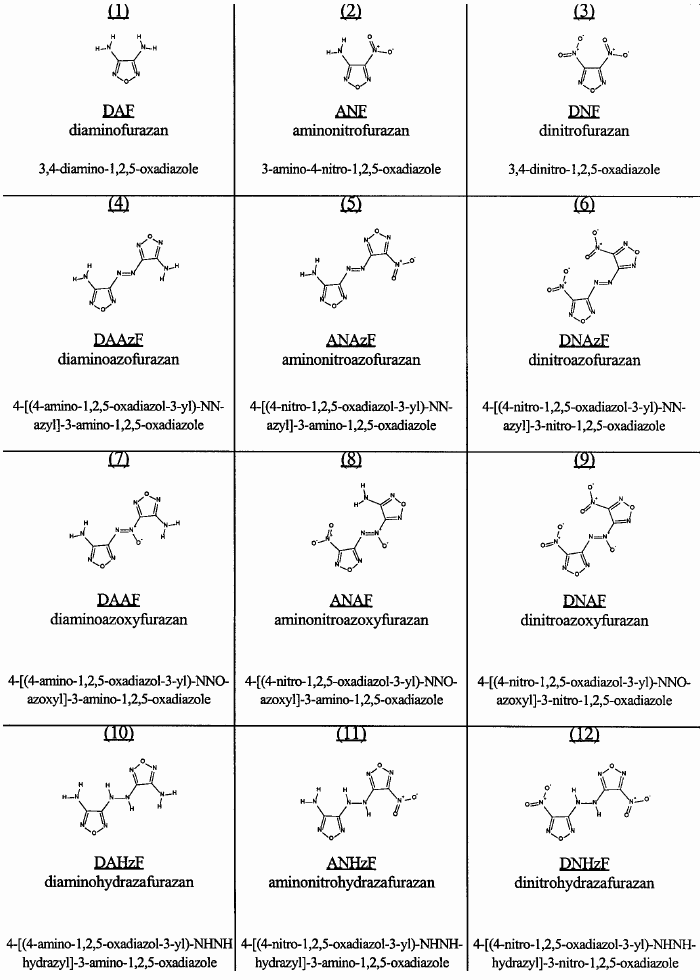
conventions. Numbers 6 & 12 in the chart below is I suppose the two mentioned compounds.
" Azido " necesarilly means the presence of an azide group - N3
and an azo group " --N=N-- in the center " is certainly not an " azoxy " group
http://en.wikipedia.org/wiki/Azoxy. hydrazo properly termed hydraza
If known , stating the number of said patent goes a long way to providing a basis for dialog ,
could this perhaps be it ? => Attachment: Furazan Derivatives US 20100132856.pdf (642kB)
This file has been downloaded 1031 times
Providing references similarly goes a long way to assessing the procedures stated.
See pdf index page 37 ( 2.12 ) , 73
Structures & Chemistry of Amino & Nitrofurazans
http://handle.dtic.mil/100.2/ADA378735
Redirects to :
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA378735&Locati...
Attachment: Review of Energetic Materials Synthesis - Furazans .pdf (135kB)
This file has been downloaded 1049 times
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I made a modest compilation of information about octonitrocubane:
https://sites.google.com/site/energeticscribble/comparison-w...
The final step uses bi-(trimethylsilyl) amino lithium as an alkaline reagent. Nitrosyl chloride is bubbled in, then treatment with ozone (oxidizing
the nitroso groups to nitro) to get eight nitro groups on the cubane cage, the yield was 55% in this step.
octanitrocubane has a heat of formation of 257.20 kcal/mol
octonitrocubane has a calculated heat of formation of 594kcal/mol , with an observed det veloc of 9.9 km/sec (at a density of 1.979 g/cm3)
Heptanitrocubane has a density of 2.028 g/cc
(there did not seem to exist a topic devoted to octonitrocubane, so this seemed like the best place to put it)
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Where are your references? Your source doesn't cite a single one!
I weep at the sight of flaming acetic anhydride.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
In Germany, Thomas Klapötke and Davin Piercey of the University of Munich, synthesized a double ringed tetrazole compound that contains a continuous
chain of 10 nitrogen atoms, with the formula C2H2N10.
The bis tetrazole joined by an azo linkage proved to be so explosive that the dry compound easily exploded, destroying glassware and setting off
further explosions as glass shrapnel hit other samples of the compound around the lab.
http://www.rsc.org/chemistryworld/News/2011/March/16031102.a...
could someone please attach the picture to this forum, in the event the link later becomes non-functional?
I think the correct chemical name for this is N,N’-azo-1,1'-bistetrazole. Please correct me if I am wrong.
The structure (in the event the attached picture becomes unavailable in the future) is
{HCN4}N=N{N4CH}
where both carbon atoms are bonded to hydrogen atoms, and the central diazo linking group is bonded to nitrogen atoms on each of the rings,
not the carbon atoms, making this compound structurally different from most of the other azo-bis-tetrazole compounds that have been prepared.
[file]14298[/file]
[Edited on 27-5-2011 by quicksilver]
|
|
|
009
Harmless

Posts: 3
Registered: 19-11-2010
Member Is Offline
Mood: No Mood
|
|
here is the image:

And youtube video
http://www.youtube.com/watch?v=Txa0fgIwiLA
"setting off further explosions as glass shrapnel hit other samples of the compound around the lab."
That is an exaggeration due to pluralization. Only one other went off.
ps. if anyone here makes this, please be careful. It is a bastard child of HMX and NI3
[Edited on 1-6-2011 by 009]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I wonder if the very same molecule but bridged by the carbon into a tricyclic compound would exist...
C2N10
Three tetrazine rings (two pentarings and one hexaring) glued together in a coplanar fashion must be much denser and so even more energetical but stil
quite unstable.

The left form would be less stable than the right one, because the chain of N atoms in the right one is shorter and that usually calls for
stability...
[Edited on 7-6-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
DicyanoDinitroMethane
Continuing the investigation of this earlier post
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
This hypothetical reaction of Malononitrile with Dinitrogen Tetroxide serves only to obtain
thermodynamic data in kilocalories for estimation. Cited values from NIST Chemistry WebBook
CH2(CN2)2 + 2 N2O4 => 2 HNO2 + / C(CN)2(NO2)2 => CO2 + 2 CO + 2 N2
+ 44.9 + 2 (- 4.7) => 2 (- 18.3 ) / + 72.1 Kcal/mol => - 94 + 2 (- 26.4 ) = - 218.9 Kcal/mol ∆He (Heat of explosion)
calculated ∆Hf (Heat of formation)
To derive a value for the density I have applied the method of H. H. Cady outlined in
LA-7760-MS , http://www.sciencemadness.org/lanl2_a/lib-www/la-pubs/003214...
Estimation of the Density of Organic Explosives from Their Structural Formulas
Density ( ρ ) is weight divided by volume ( mol weight )( k ) / V
1 5 6. 0 7 x 0.7686 / 6 9. 0 8 Derived value comes to ρ = 1.736 gm/cm³
Dividing the ∆He Heat of explosion of 1 mol of the compound by it's molar weight , - 2 1 8.9 / 1 5 6.07
obtains ∆He Heat of explosion = - 1 4 0 2 Kcal per kilogram of compound ' Q '
To estimate the Detonation pressure generated and Velocity of Detonation ,
I have applied the method of M.J. Kamlett, S.J. Jacobs outlined in
The Journal of Chemical Physics , vol 48 , num 1 , http://handle.dtic.mil/100.2/AD661483
A Simple method for Calculating Detonation Properties of C-H-N-O Explosives
Values for M is 36 , for N it's 0.256
Detonation pressure = P = 15.58 ρ ² ( N √ M √ Q )
P = 270 Kilobar
Velocity of Detonation = V = 1.01 ( 1 + 1.3 ρ ) √( N √ M √ Q )
VOD = 7880 Meters/sec
Complete procedure detailed in this post
http://www.sciencemadness.org/talk/viewthread.php?tid=11195#...
Please observe subsequent correction noted here
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
------------------------------------------------------------------------------------------
C-H Bond Dissociation Energy of Malononitrile
http://www.chem.arizona.edu/sanov/pdf/jz900379treprint.pdf
On the Non-Additivity of pK Values of Polynitromethanes
http://abulafia.ciencias.uchile.cl/publicaciones/pdf/12-Tetr...
A Comparative ab initio Study of the Dicyanomethanide, Cyanonitromethanide, Dicyanamide and Cyannitramide Anions
http://actachemscand.dk/pdf/acta_vol_31a_p0151-0154.pdf
------------------------------------------------------------------------------------------
Curiously the anticipated elevated performance one might expect of a cyano - nitro compound
is not realized upon analysis by Kamlett / Jacobs. This excercise demonstrates the value of
ab initio projection of performance. Interestingly by comparison the detonation utility of forum
member ' Engager ' estimates this much higher.
http://www.sciencemadness.org/talk/files.php?pid=169218&...
- * Please cite any apparent error which may account for this wide discrepancy.
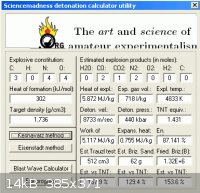
HE calc by ' enhzflep ' similarly , although this tends to overstate levels of performance
http://www.sciencemadness.org/talk/files.php?pid=64852&a...
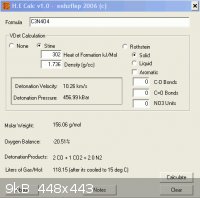
------------------------------------------------------------------------------------------
Formation of this hypothetical structure might be realized by displacing the Chlorine
of Dicyanonitrochlormethane C(CN)2(NO2)Cl, possibly by solvation in DMSO with
Sodium Nitrite. C(CN)2(NO2)Cl + NaNO2 => NaCl + C(CN)2(NO2)2
Dicyanonitrochlormethane C(CN)2(NO2)Cl itself is made from Potassium Cyanide and Chloropicrin
( KCN + CCl3NO2 ) as described here :
A Dictionary of Chemistry and the Allied Branches of Other Sciences Vol 6 1872
Dicyanonitrochloromethane , page 446
http://books.google.com/books?id=sKktAAAAYAAJ&pg=PA446&a...
Chloropicrin heated with potassium cyanide, alcohol, and water, is converted
into dicyano-nitro-chloromethane, C(NO2)Cl(CN)2 This compound is soluble in
water, alcohol, ether, and chloroform, very easily decomposible, and has not
been obtained in the separate state, but only in combination with water, and
with metallic oxides and salts. Lead acetate added to its aqueous solution
forms a prescipitate obtaining C(NO2)Cl(CN)2 • 3PbO ; with silver nitrate a
precipitate is formed consisting of 3C(NO2)Cl(CN)2 • 4NO3Ag • 8H2O
For the details of the preparation, which requires particular precautions
we must refer to the original paper. On a Cyanogen Derivative of Marsh Gas
( Bassett, Chem. Soc. J. [2] iv. 352 ). Journal of the Chemical Society ( J. Chem. Soc.)
Many thanks to forum member ' gsd ' for providing this paper. - Attachment: Dicyano nitrochloro methane.pdf (110kB)
This file has been downloaded 844 times
Chloropicrin may readily made in at least two ways described below.
http://www.sciencemadness.org/talk/viewthread.php?tid=3214&a...
http://www.youtube.com/watch?v=OxPoWZJW20o
http://www.sciencemadness.org/talk/viewthread.php?tid=710
A Bibliography of Chloropicrin 1848-1932
http://books.google.com/books?id=sq4oAAAAYAAJ&lpg=PA19&a...
Chloropicrin by Aqua regia on Acetone

--------------------------------------------------------------------------------
http://history.amedd.army.mil/booksdocs/wwi/VolXIV/VolXIVhtm...
CHLOROPICRIN
Chloropicrin, CCl3NO2, is a colorless liquid, boiling at 112 °C, and having a vapor pressure of 5.8 mm.
at 0 °C, 14.0 mm. at 15 °C, and 23.8 mm. at 25 °C. The vapor is nearly six times as dense as air. The
density of the liquid is 1.6924 at 4 °C and 1.6539 at 20 °C, the two determinations not being made by
the same man. The melting point is - 69.2 °C. Chloropicrin is not sufficiently volatile for use by itself in
cloud attacks. While it has been used mixed with 75 percent chlorine, it was usually fired in shell. It is
moderately toxic, 0.8 mg. per liter (110 p.p.m.); somewhat lacrymatory, 0.016 mg. per liter, and liable
to cause vomiting, thus forcing removal of the mask. It was not stopped satisfactorily by the charcoal
first used in the masks. The laboratory charcoal eventually employed was about one thousand times as
effective as the earlier material. Chloropicrin is practically nonmiscible with water, and a mixture of the
two boils at about 84 °C. It is miscible in all proportions with many organic solvents. There is a marked
evolution of heat when it is mixed with methyl alcohol, ether, or acetophenone; a slight evolution of
heat when mixed with isobutyl alcohol, isoamyl alcohol, or carbon bisulphide.
Chloropicrin is not hydrolyzed by water, nor by cold hydrochloric, sulphuric, or nitric acid. When heated
with these acids it is said to distill unchanged. Dilute aqueous sodium hydroxide does not attack it; but
alcoholic sodium hydroxide decomposes it slowly, and sodium ethylate attacks it fairly readily, forming
the orthocarbonic ether, CCl3NO2 + 4C2H5ONa = C(OC2H5)4 + 3NaCl + NaNO2. Chloropicrin can be
heated for several days with aqueous ammonium hydroxide at 100 ºC without undergoing any
appreciable change. At 150 ºC, or when heated with alcoholic ammonia, a reaction takes place in a few
hours, guanidine being formed, HN:C: (NH2)2. Alcoholic potassium acetate decomposes chloropicrin
completely at 100 ºC and alcoholic potassium cyanide reacts at a lower temperature, the product in
this last case having the formula (CN)2C(NO2)-Cl.. Though chloropicrin is attacked very slowly by dilute
aqueous sodium hydroxide, it unites readily with neutral potassium sulphite,
CCl3NO2 + 3K2SO3 + H2O = CH(NO2)(S03K)2 + 3KCl+KHSO4.
http://cameochemicals.noaa.gov/chemical/387
http://www.cdc.gov/NIOSH/ershdb/EmergencyResponseCard_297500...
http://www.cdc.gov/niosh/idlh/76062.html
http://www.cdc.gov/niosh/npg/npgd0132.html
http://pmep.cce.cornell.edu/profiles/extoxnet/carbaryl-dicro...
http://en.wikipedia.org/wiki/Chloropicrin
Attachment: Chloropicrin MSDS.pdf (113kB)
This file has been downloaded 1202 times
Attachment: Chloropicrin label use.pdf (21kB)
This file has been downloaded 873 times
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A closely related compound Ammonium dinitroacetonitrile NH4C(NO2)2CN has crystal density about 1.8 g/cc




Chemistry of dinitroacetonitrile I
http://www.sciencedirect.com/science/article/pii/S0040402001...
Chemistry of dinitroacetonitrile II
http://www.sciencedirect.com/science/article/pii/S0040402001...
Chemistry of dinitroacetonitrile III
http://www.sciencedirect.com/science/article/pii/S0040402001...
Chemistry of dinitroacetonitrile IV
http://www.sciencedirect.com/science/article/pii/S0040402001...
Lead-Free Initiator Materials for Small Electro-Explosive Devices
for Medium Caliber Munitions Final Report 04 June 2003
www.dtic.mil/dtic/tr/fulltext/u2/a438486.pdf
J. B. Christian, Nav. Ord. 3387 N.O.L. (1954) p. 23.
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Explosives research has proceeded principally by inspiration of the researchers.
In recent times exhaustive analysis of every conceivable or imaginable molecule
has become feasible with the advent of computers running algorithms to compile
encyclopedic ab initio data identifying promising candidate structures. The problem
with this approach is that once a particular molecule deemed worthy of research
is identified , the way and means of it's synthesis remains to be speculated about ,
since the programs only consider functional groups and moieties not reaction
schemes. In my view it may be more productive to look for precursors that can
readily host explosophore functional groups using well established reaction schemes.
If you 're wondering at the practicality of this approach , this is how Edison developed
his light bulb , literally going to the haystack and sampling every straw until he found
the needle - 17000 experiments later. He was grossly understating the problem
when he quipped " invention is 1 % inspiration and 99 % perspiration ".
In a related post I outlined the range of possible base adducts applicable for addition
compounding here => http://www.sciencemadness.org/talk/viewthread.php?tid=13174#...
Threads in the references section citing papers on investigatory research into new
explosives compounds.
http://www.sciencemadness.org/talk/viewthread.php?tid=7518&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=7518&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=7518&a...
PHILOU Zrealone outlined here candidate functional groups for energetic compounds
http://www.sciencemadness.org/talk/viewthread.php?tid=1778#p...
 
Summarized in the chart above is the seven reaction schemes depicted above it. The
varied products highlight the range of possible outcomes from a few precursors. The
order in which reagents are introduced in a reaction scheme affects the result obtained,
what is produced and the yield of product. Exhaustively investigating every conceivable
interaction of selected reagents can yield many new and unexpected results.
What this does is provide a framework for rigorous systematic evaluation.
Of course many schemes will not yield any useful results.
______________________________________________________________
When we are concerned with a group as a set without reference to any order, it is a problem in combination.
A permutation is a combination of objects that has also an order or sequence of arrangement imposed.
There is only one group combination , A, B, C, of that there can be 3 groups A, B, , A, C, , B, C, each in turn can
have 2 permutations ( A, B, or B, A,) ( A, C, or C, A,) ( B, C, or C, B,) adding the missing letter to each we have
A, B, C, , A, C, B, , B, A, C. , B, C, A, , C, A, B, , C, B, A, , note that none repeat an order.
If there are 9 horses in a race and only the first 3 to finish pay off , how many groups of winners can there be ?
9 x 8 x 7 = 84
3 x 2 x 1
How many ways are there in the order those 84 groups can finish ? 9 x 8 x 7 = 504
since the ways to place 1st , 2nd , 3rd of any particular group is 3 x 2 x 1 = 6 , then 6 x 84 = 504
_______________________________________________________________
There is only one combination of the 5 reagents but 120 possible permutations of the
order in which they can be reacted. There are 5 possible combinations of 4 of the reagents
with 24 possible permutations of the order in which each of those can be reacted.
10 possible combinations of any 3 of the listed reagents having 6 permutations each.
10 combinations of any 2 reagents , with some exceptions order matters little at this point.
U - urea , F - formaldehyde , G
- glyoxal , S - sulfamate , N - nitromethane
- the combinations of groups of 3 out of the 5 -
UFG , UFS , UFN , UGS , UGN , USN , FGS , FGN , FSN , GSN
- and their permutations -
UFG , UGF , GUF , GFU , FUG , FGU
UFS , USF , SUG , SFU , FUS , FSU
UFN , UNF , NUF , NFU , FUN , FNU
UGS, USG , SUG , SGU , GUS , GSU
UGN, UNG, NUG , NGU, GUN , GNU
USN , UNS, NUS , NSU , SUN , SNU
FGS , FSG , SFG , SGF , .GFS. , GSF
FGN , FNG, NFG , NGF , .GFN , GNE
FSN , FNS , NFS , NSF , .SFN , SNF
GSN, GNS, NGS , NSG , SGN , SNG
__________________________
- the 5 groups of 4 combined reagents out of the 5 -
UFGS , UFGN , UFSN , UGSN , FGSN
- and now their permutations -
UFGS , FUGS , FGUS , FGSU
UFSG , FUSG , FSUG , FSGU
UGFS , GUFS , GFUS , GFSU
UGSF , GUSF , GSUF , GSFU
USFG , SUFG , SFUG , SFGU
USGF , SUGF , SGUF , SGFU
UFGN , FUGN , FGUN , FGNU
UFNG , FUNG , FNUG , FNGU
UGFN , GUFN , GFUN , GFNU
UGNF , GUNF , GNUF , GNFU
UNFG , NUFG , NFUG , NFGU
UNGF , NUGF , NGUF , NGFU
UFSN , FUSN , FSUN , FSNU
UFNS , FUNS , FNUS , FNSU
USFN , SUFN , SFUN , SFNU
USNF , SUNF , SNUF , SNFU
UNFS , NUFS , NFUS , NFSU
UNSF , NUSF , NSUF , NSFU
UGSN, GUSN, GSUN , GSNU
UGNS, GUNS, GNUS , GNSU
USGN, SUGN, SGUN , SGNU
USNG, SUNG, SNUG , SNGU
UNGS, NUGS, NGUS , NGSU
UNSG, NUSG, NSUG , NSGU
FGSN, GFSN , GSFN , GSNF
FGNS, GFNS , GNFS , GNSF
FSGN, SFGN , SGFN , SGNF
FSNG, SFNG , SNFG , SNGF
FNGS, NFGS , NGFS , NGSF
FNSG, NFSG , NSFG , NSGF
Ths only assumes a simple serial addition.
This does not consider the mixing of pairs
of already premixed reagents , for example
NSGF seen immediately above here can
also be arrived at in an altered reaction
scheme by adding to NS , GF.
.
[Edited on 9-7-2011 by franklyn]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I disagree. While investigating every possible permutation of target molecule and testing the result is productive, though time consuming, researchers
do and should save much time and effort by just ignoring combinations that are fairly certain not to yield desirable properties. Trying consider every
permutation of reaction order of addition would just be a big waste of time, even with sophisticated computer programing. Knowledged intuition is
essential to designing, and synthesizing, new better energetic compounds. While many compound have been discovered by accident, the increasing
complexity of molecular structures and synthesis makes it extremely unlikely now that any advances can be made relying on chance. All the low hanging
apples have already been plucked from the tree, so to say.
Energetic research could be advancing much more rapidly if there was more research funding to attract talented chemists, but as it is, there are only
about 25 energetic researchers in the USA, and half of those do not even do research full time. I have a feeling that often they simply give up on
target many molecules because they cannot figure out a workable synthesis, or do not care enough to hire a highly knowledged consultant.
Personally, I would like to see some research done on the target molecules I designed.
http://www.shadowrx.com/forums/showthread.php?t=1505
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ AndersHoveland
Lonely molecules left unrealized does not comprise useful practice of effort.
You are assuming an omniscience which does not exist nor can it be possible.
Just as the practice of integration in calculus involves the use of references
that detail known integrals - because their derivation is otherwise practically
unrealizable.
The common constituent of most of the now over 50,000,000 identified molecules
is the aromatic carbon ring , yet less than 1000th of one percent would ever
involve benzene as a starting material in their synthesis. As an example if Mesitylene
were a target how would one naively approach this ? The counterintuitive reaction
of acetone and sulfuric acid likely would not spring to mind. Reaction schemes are
what is wanting , not the target molecules.
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I have found a new N10 molecule.
This is from Wikipedia:
"Chlorine azide is prepared by passing chlorine gas over silver azide or by an addition of acetic acid to a solution of sodium hypochlorite and sodium
azide.[3]
When treated with ammonia it is conceivable that one or more of the three possible azinamines, NH2N3, NH(N3)2, and N(N3)3 may be formed."
N(N3)3 is mentioned. But I can't find any other info about it. Any ideas?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 12332123  | | Or, if one doesn't mind hyperactive sensitivity, aminotetrazolium nitroformate could be synthesised. This has perfect oxygen balance and would likely
beat HMX by a fair margin (IIRC the dinitramide salt has a detonation velocity ~9500) |
I do not think aminotetrazolium nitroformate would be particularly sensitive. Ammonium nitroformate is very stable, and even hydrazinium nitroformate
is stable enough for use as a rocket fuel oxidizer. Hydrazinium nitroformate has a friction sensitivity of 25-36 N, compared to 120 N for RDX.
Aminotetrazolium nitrate is also apparently less sensitive than RDX. This information leads me to believe that aminotetrazolium
nitroformate would be a relatively insensitive explosive, with a very high detonation velocity (probably >9km/sec).
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I have an idea for new explosive. Here is the picture and possible synthesis:

|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Just when you thought azidotetrazole couldn't get more energetic somebody (guess who) attaches an oxygen to it making salts which are less sensitive
and more powerful (azidotetrazolate 2-oxide):
http://onlinelibrary.wiley.com/doi/10.1002/chem.201102064/ab...
|
|
|
| Pages:
1
..
4
5
6
7
8
..
23 |
|