| Pages:
1
2 |
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
tryptophan to alpha methyl tryptamine
before i get started
http://en.wikipedia.org/wiki/Alpha-Methyltryptamine
"It is legal in the United Kingdom, however, and does not fall under the tryptamine clause as its substituent is not on the nitrogen position"
I live in the UK, do not give me unjust prejudice, purely theoretical
is this logical?
I was just messing around with the tautomerism function with various molecules and i saw a possible route, thanks for any non hate-incited comments
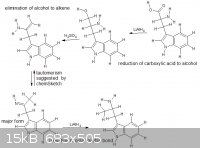
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
For the elimination step, I think there would be a hydride shift in favor for the conjugated pi system as the major product. Correct me if I'm wrong
here.
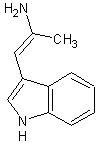
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
The elimination step will not work. You obviously never worked with tryptamines.  First of all, see the abundant discussion on amphetamine from phenylalanine (Also search for phenylalanine at https://www.erowid.org/archive/rhodium/chemistry/index.html and have a look at the referenced literature.). IMO, nothing kitchen-chemistry
friendly every came out of that. If I had to, I would try decarboxylation, oxidation to the nitrile, Grignard with MeI and reduction of the imine
intermediate. But I doubt that it would work, so I would use the tryptophan for DMT and try a route from indole, which is available and may be doable
in the ghetto. First of all, see the abundant discussion on amphetamine from phenylalanine (Also search for phenylalanine at https://www.erowid.org/archive/rhodium/chemistry/index.html and have a look at the referenced literature.). IMO, nothing kitchen-chemistry
friendly every came out of that. If I had to, I would try decarboxylation, oxidation to the nitrile, Grignard with MeI and reduction of the imine
intermediate. But I doubt that it would work, so I would use the tryptophan for DMT and try a route from indole, which is available and may be doable
in the ghetto.
Edit:
PS: Please don't post pictures with an alpha-channel. For whatever reason my browser uses a black background for such images. Therefore I had to
remove the alpha-channel with an image manipulation software. Also for clarity remove the H-atoms from the structure images, as is the norm in organic
chemistry.
[Edited on 15-5-2012 by turd]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
thanks sargent1015, i cant correct you or even debate on it, turd thanks for the reply, i had a look and came up with a new theoretical synth, however
"doable in the ghetto", im not intending on a practical synthesis, i hope the new image is up to your standards however i dont know what alpha is
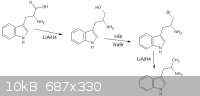
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
thanks sargent1015, i cant correct you or even debate on it, turd thanks for the reply, i had a look and came up with a new theoretical synth, however
"doable in the ghetto", im not intending on a practical synthesis, i hope the new image is up to your standards however i dont know what alpha is
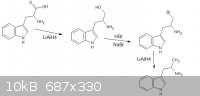
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
decarboxylation is the way to start but it's not a ghetto synth because of the expensive solvent required. on the other hand maybe you could run the
decarboxylation in a silicone oil. I'd hate the workup...
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Synthetic chemistry is the science of reactions that work in practice. "Theoretical synths" belong to the realm of science fiction. Not uninteresting,
but also not the topic of this particular message board.
Given a standard textbook on organic chemistry and considering in each step of the synthesis only one functional group, while ignoring all others,
everybody can come up with the most fabulous "theoretical synths". There is no value to that.
To establish a basis of discussion it is your duty to find literature demonstrating the validity of your approach, namely the reduction of an
alpha-aminoacid to the methyl-derivate via an intermediate bromide without protection groups. If you found that, we can discuss whether it is viable
for this particular substrate.
If you don't know how to perform a literature search, you will find excellent and comprehensive guidelines in "Forum matters".
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | | If you don't know how to perform a literature search, you will find excellent and comprehensive guidelines in "Forum matters". |
In this post.
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
well the molecule is legal so i will demonstrate validity if i can get hold of/make lialh4
[Edited on 16-5-2012 by rannyfash]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I spoke too soon: lot of good ways to decarboxylate- http://www.erowid.org/archive/rhodium/chemistry/tryptophan.h...
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
is there any problems with HBr or LiAlH4 and indole derivatives?
|
|
|
Alastair
Hazard to Self
 
Posts: 59
Registered: 13-7-2011
Member Is Offline
Mood: Barely any solvent in my emulsion
|
|
I think HBr could carry some water and it is generally an unwanted thing in indole syntheses, but i may be wrong, what do you guys think?
There are even harder methods (?) where the =o group is halogenated first, then OH reduced with LAH.
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Correct me if I am wrong, but would it be possible to do a Kochi on tryptophan using Pb(OAc)4 and LiBr/LiCl to the halide, then a Grignard with MeBr?

|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
I'm not sure the Kochi reaction would work on tryptophan in the first place, but even if it did the product would have an amine on the same carbon as
a halogen. These types of molecules are usually very unstable and it would almost certainly decompose into an imine with expulsion of the halogen.
Depending on the workup conditions this imine could also be destroyed by being hydrolyzed to the aldehyde. Again this is all assuming that the Kochi
reaction was successful.
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
could protecting groups be introduced to prevent immine decomposition? however any substituent would make the practical procedure illegal in the uk
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
To prevent decomposition TO the imine (elimination)? Or to prevent decomposition OF the imine (hydrolysis)? Either way protecting groups won't really
help you, so no. If you're dead set on a synthetic route from tryptophan to alpha-methyltryptamine there are certainly more practical reactions to
look at, modify to fit your target, and try. As far as kitchen chemistry goes it doesn't get much more simple than mixing two or three things together
and then stir/heat until your product separates or precipitates out. So i'll do you a favor and put forth a marginal amount of effort to find some
reactions along these lines that might be able to be tweaked to fit your goal.
Trying to turn phenylalanine into amphetamine is analogous to what you're trying to do. So with that in mind this is what I found.
Phenylalanine, when mixed with TCCA and aqueous NaOH, gives almost quantitative yields of phenylacetonitrile which essentially separates from the
solution.
https://docs.google.com/viewer?a=v&q=cache:a514FLGr4D8J:...
If tryptophan were used instead of phenylalanine, it is very likely that indole-3-acetonitrile would be produced.
And since phenylacetonitrile can give a direct precursor to amphetamine (phenyl-2-propanone) in two steps
http://www.erowid.org/archive/rhodium/chemistry/phenylaceton...
Perhaps the same would hold true for indole-3-acetonitrile and alpha-methyltryptamine. The acetylation isn't exactly "kitchen chemistry", but if you
can't find a way to make that work I doubt you should be handling large quantities of something as potentially hazardous as AMT anyways. It's also
worth noting that although it may be easier to view those two procedures side by side, both the acetylation and hydrolysis/decarboxylation have been
taken from a site called www.orgsyn.org. This is a wonderful site full of great procedures and references and if you wish to continue to learn more about organic chemistry
I highly encourage you to have a look around there.
In the end, am I sure these procedures will work for your goals? No. Why? Because seeing a reaction work on one molecule doesn't immediately mean that
it will work on another molecule just because they happen to look similar in some way. If you really are intent on finding a way to make this work,
and it is legal in your country as you have said, then by all means give it a shot. And if you really want to contribute to this forum then please
post your procedure and results for us to examine.
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
why not producing the carbonylchlorid of tryptopahn with SOCl2 and reducing it to the aldehyd via Rosenmund reduction. Afterwards you do a
Wolf-Kishner on the aldehyd to yield the desired amino alkane.
best regards
[Edited on 29-5-2012 by Organicus]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
my idea was to have carbamate groups on the amine to prevent imine formation but i doubt will work rethinking about it, if the imine did form could
the electrophillic imine bond attack a methyl carbocation under any circumstances?, i liked your synth, i haven't really looked into amphetamine but
it seems like a viable analogous route, i also made a nice little diagram :3, i cant try it atm im focusing all my efforts on revision for my A2 level
exam in two weeks
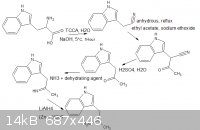
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Ohhh.... Niiiice! If someone can make it work on tryptophan, this would be quite a break-through. There are no excuses - all of these reagents can be
trivially sourced.
Oh no! IMHO this is an ugly route to phenylacetone. And how rude to remove the nitrogen atom which is already in the right position! A more classy
route is given on the same page: react with MeMgI and reduce the intermediate imine salt in one pot. If this works on indol-3-ylacetonitrile (a second
big if) then you could also make the alpha-ethyltryptamine in two steps from tryptophan. 
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
Yes the MeMgI route would certainly be simpler for most, but as the original poster seems to have a limited amount of experience in the lab I decided
not to suggest the use of grignard reagents.
In case anyone is wondering what turd and I are referring to, here is the procedure:
http://www.erowid.org/archive/rhodium/chemistry/amphetamine....
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
"Phenylalanine, when mixed with TCCA and aqueous NaOH, gives almost quantitative yields of phenylacetonitrile which essentially separates from the
solution."
Im sorry but this synthesis was far away from quantitative! I carried it out once and it was a mess. Also the free -CN polymerizes fast in the
solution so the yield is poor. It was my first try on this reaction so im sure it could be carried out smoother. I didnt weigh the benzylcyanide, but
i hydrolised it with H2SO4 and vaccuum destilled the phenylacetic acid. yield was around ~10-15% based on pheylalanine. To prevent polymerisation of
the cyanide i added a non polar solvent to the mix in a later stage of the reaction. Its very important to properly wash the filter cake afterwards,
there is the biggest part cyanid present.
PS: Only try this reaction if youre in possession of a really good fume hood, Chlor gas is really nasty and wouldnt let you breath near the reaction
vessel. Eyes are irritated of course too.
Also essential is a overhead stirrer due to the sirup like consistency.
best regards
[Edited on 29-5-2012 by Organicus]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
"Emulsifier (soap), 2-propenenitrile, various butadiene monomers (including 1,3-butadiene, 1,2-butadiene), radical generating activators, and a
catalyst are added to polymerization vessels in the production of hot NBR. Water serves as the reaction medium within the vessel. The tanks are heated
to 30–40 °C to facilitate the polymerization reaction" taken from good old wiki in nitrile rubber, describing how polymerisation is deliberately
carried out
did you keep the reaction below 5 celcius as suggested? were your reagents pure(contaminant catalyst)? 10-15% yield, 100g tryptophan is relatively
cheap, active doses on mice are in the microgram quantity's, im trying to work a new way
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
yes the reaction was cooled properly from the out- and the inside. the reaction vessel stood in a NaCl/Ice/H2O bath the whole time. some chunks of
pre-frozen dest. H2O where thrown into the solution and got exchanged when they were molten. the reagents were pure except the TCCA, it was
"contaminated" with some CuSO4 (tcca for swimming pools). But that shouldnt have been an issue, right?
Yes of course 10% of 100g is a lot when it comes down to micrograms none the less: yield sucks =)
best regards
PS again: The freed benzylcyanide were yellow blobs of oil in the solution which became brown sludge in about 5-10 min, while the reaction took place.
And the problem is one cant just stop to deal with it because there is massivly building up foam. thats the second reason why a good overhead stirrer
is needed, but youll see =)
Sadly even alpha-methyltryptamine is restricted in my country so i couldnt do my research on that field. =(
[Edited on 29-5-2012 by Organicus]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
thanks, also the picture, i think this is a method that is easier when compared to grignard reagents but certainly not safer, organolithium compounds
are the most awesome thing i have ever made, (in preparation of a miniscule amount of LiAlH4) and i have no idea if any of these mechanisms will
actually work, i need to test it, i stole the formic acid mechanisms from http://en.wikipedia.org/wiki/Eschweiler%E2%80%93Clarke_react...
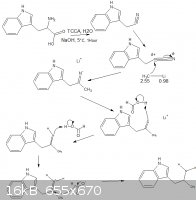
[Edited on 29-5-2012 by rannyfash]
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | | thanks, also the picture, i think this is a method that is easier when compared to grignard reagents but certainly not safer, organolithium compounds
are the most awesome thing i have ever made, (in preparation of a miniscule amount of LiAlH4) and i have no idea if any of these mechanisms will
actually work, i need to test it |
im trying to avoid alkylating agents because of their carcinogenic properties, but im looking forward to your writeup!
in my eyes the reaction youve drawn will stop at the imine, i dont think there is a hydrogenation of the imine with formic acid.
best regards
[Edited on 29-5-2012 by Organicus]
|
|
|
| Pages:
1
2 |