Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
calcium nitriminotetrazolate: a safe(er) primary explosive
Figured a primary explosive with an impact sensitivity much lower than RDX, and a comparable friction sensitivity would be of interest here.
http://www.explosci.com/calcium-nitriminotetrazolate/
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Sounds rather promising. Relatively long synthesis, if you have not ATZ as a start point. And dehydration- 150 Celsius under vacuum may be a little
problematic in kitchen.
Women are more perilous sometimes, than any hi explosive.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Check out this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The barium salt or other salts may have interest. Some of these compounds could also be of interest in mixture with Ethyleneditetrazylazide see page
1,
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
as a plastique possibly having sensitivity properties which could be adjusted to whatever sensitivity is desired by varying the percentage of the oily
liquid Ethyleneditetrazylazide.
Also see these
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
[Edited on 18-5-2012 by Rosco Bodine]
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
44 page pdf on various salts including calcium nitiminotetrazolate:
See page 12 for chemical structure, experimental synthesis and chemical and explosive properties.
[Edited on 19-5-2012 by Sickman]
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
That description of the synthesis of nitriminotetrazole is odd. that stuff is definitely sensitive towards a hammer blow!
Product sounds more like aminotetrazolium nitrate.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Explosci  | That description of the synthesis of nitriminotetrazole is odd. that stuff is definitely sensitive towards a hammer blow!
Product sounds more like aminotetrazolium nitrate. |
I later concluded that is what it should be, though haven't identified it. The synthesis was an experimental attempt to replace pure nitric acid with
red fuming nitric acid under non-anhydrous conditions (which related to the method of Klapoetke mentioned earlier in the thread). It was not a
success.
The method of shock sensitivity testing initially wasn't the best, the thought was aluminium would help prevent the powder from spreading during
impact which was the case, but this also made the test ineffectual. The mechanical impact force was transferred through the aluminium, so that even
very sensitive material would not show a reaction under that circumstance.
The yellow silver salt described there makes big booms. It has brisance like or maybe better than silver nitrotetrazolate when exploded against
aluminium at similar amounts. However it possess a low sensitivity to or is relatively insensitive to flame, heat and shock. Not ideal properties for
a detonant. But still good for demonstration when heated over a flame for quite some time. I still don't know what this salt is.
I also hadn't realized cuprous nitrotetrazolate (DBX-1) was so sensitive. It is apparently significantly more impact sensitive than lead azide. There
seems to always be a minus when replacing one initiator for the other, especially lead azide.
I'm wondering what weaknesses of calcium nitriminotetrazolate could be. If it is so insensitive to shock, I would reckon it is not as flame sensitive
as lead azide. Lead azide has superb flame sensitivity, something seen in only a few good primaries, many of them more sensitive than itself. Water
solubility could be an issue.
If you are feeling more risky for an initiator to experiment with you could try silver azotetrazolate in the smallest amounts. It has high brisance
and extreme sensitivity, and is just all around a grumpy customer. It has the highest sensitivity to static shock I've ever seen in an initiator.
I wonder now if sodium azoxytetrazolate pentahdyrate could act as a detonant (sodium salt detonator), it seems to have some brisance and flame
sensitivity. Mediocre low water solubility, and it does not react to hammer blows. Though it might still have some static issues.
[Edited on 21-5-2012 by Formatik]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
One of the nitriminotetrazole compounds that would definitely be worth looking at would be Stabanate. Also an experiment of interest is perhaps a
possible nickel analogue of Stabanate if it exists. Or in the alternative, analogous to the double salt involving nickel styphante and potassium
styphnate, similarlly the potassium nitriminotetrazolate and nickel nitriminotetrazolate, or a styphnate nitriminotetrazolate mixed salt of nickel.
I believe the potential for such possible multiple salts exists.
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
Quote: Originally posted by Formatik  |
I later concluded that is what it should be, though haven't identified it.....
The yellow silver salt described there makes big booms. It has brisance like or maybe better than silver nitrotetrazolate when exploded against
aluminium at similar amounts. However it possess a low sensitivity to or is relatively insensitive to flame, heat and shock. Not ideal properties for
a detonant. But still good for demonstration when heated over a flame for quite some time. I still don't know what this salt is.
|
Sounds like disilveraminotetrazolium nitrate
http://www.wydawnictwa.ipo.waw.pl/cejem/3-2010/PDF/Delalu.pd...
Quote: Originally posted by Formatik  |
I also hadn't realized cuprous nitrotetrazolate (DBX-1) was so sensitive. It is apparently significantly more impact sensitive than lead azide. There
seems to always be a minus when replacing one initiator for the other, especially lead azide.
|
From what reference?
http://www.dtic.mil/ndia/2010armament/ThursdayReunionMichael...
go to page 11. only slightly more sensitive than lead azide
Quote: Originally posted by Formatik  |
If you are feeling more risky for an initiator to experiment with you could try silver azotetrazolate in the smallest amounts. It has high brisance
and extreme sensitivity, and is just all around a grumpy customer. It has the highest sensitivity to static shock I've ever seen in an initiator.
|
static sensitivity is freaky high of the dry silver salt. Had this stuff go off a bunch of times. Worse than silver fulminate
[Edited on 22-5-2012 by Explosci]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I have considered it to be disilveraminotetrazole nitrate, but as that reference points out this is a white powder, and it is a "very sensitive
compound!!" also according to the same reference. But, impact sensitivity is given as 15 J. Strange they call it very sensitive, considering RDX is 7
J. Down more in their notes 3 J is considered "very sensitive" for impact. This is about what lead azide is (2.5-4.0 J) and it is one of the least
impact sensitive primaries in use.
Color variations can be a bother when we consider, for example, the different colors reported for cupric azide in the literature, but it can also
still indicate a different compound. If being able to withstand violent hammer blows on an iron plate is what 15 J is all about, perhaps the yellow
silver salt salt is indeed nothing more than a colored variant of the complex (or still something else).
| Quote: | | From what reference? |
There was this reference below I was reading showing it might be more impact sensitive. Well ok, more or less more sensitive according to the
deviations.
DBX-1 – A Lead Free Replacement for Lead Azide.
http://onlinelibrary.wiley.com/doi/10.1002/prep.201100056/pd...
| Quote: | | static sensitivity is freaky high of the dry silver salt. Had this stuff go off a bunch of times. Worse than silver fulminate
|
I've not had any incidents with these salts, and I also compared the two. Silver fulminate and silver azotetraozlate are quite similar in some
regards. Some notable differences I've seen: azotetrazolate was less friction sensitive (still very high), but a lot more brisant. The latter part is
what makes it the riskier compound in my opinion.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Actually, the charge distribution of the nitriminotetrazolate anion more closely resembles that shown in the below resonance structure, explaining the
great stability of nitriminotetrazolate.
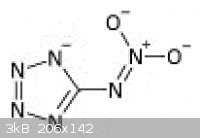
The tetrazole ring is actually aromatic (like benzene), whether or not it is in its ionic form. The electron charge is distributed over all
four nitrogen atoms.
Another reason for the low sensitivity of calcium nitriminotetrazolate is probably that it is a salt composed of +2 charged cations and -2 anions,
giving a high crystal lattice energy. For the same reason, such salts tend to have very low solubilities, suggesting good stability against moisture.
The reason that Cu, Ag, Hg, Pb salts tend to be more sensitive is because the bonds are more covalent, meaning there is not an extra electron
available to stabilize the explosive cation. The Ag+1 ion is especially oxidizing, so its salts are usually the most sensitive.
[Edited on 25-5-2012 by AndersHoveland]
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  |
Another reason for the low sensitivity of calcium nitriminotetrazolate is probably that it is a salt composed of +2 charged cations and -2 anions,
giving a high crystal lattice energy. For the same reason, such salts tend to have very low solubilities, suggesting good stability against moisture.
[Edited on 25-5-2012 by AndersHoveland] |
Sorry, but that's not true. Situation is just opposite. To get rid of water (after preparation calcium salt of nitriminotetrazolate contents 5
molecules of H2O) one needs to heat aforementioned salt up to 150 Celsius under vacuum. It means definitely that this salt is very hygroscopic like
many salts of calcium.
Women are more perilous sometimes, than any hi explosive.
|
|
|