CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
Exotic Oxidizers
Hello,
I am making (have made some already) exotic oxidizers which will burn fast when mixed with a reductor (Mg,Al,Zn). Here is my list at the moment:
The salts (mostly nitrates & (per)chlorates) of:
Ammonium, Aluminium , Potassium, Sodium (only Chlorate and nitrate yet!), Gallium, Tin, Magnesium, Zinc, Lead, Iron.
The list isn't big at all and most of them aren't actually "exotic".
The most common oxidizers in pyrotechnic flash powders are just potassium (per)chlorate & nitrate. But I think that there are alot more powerful
oxidizers (Like the gallium chlorate) which are so unknow but give beautifull results.
If you would know more that are able to make from common chemicals (can get concentrated nitric, sulfuric & hydrochloric acid and most common
chemicals) please let me know even if you don't know a synthesis I will try to find it.
Will make soon a list on how to make all those exotic oxidizers.
(P.S: With exotic I mean : 1. Not much information to find of on google 2. (Almost) none youtube videos about it. 3: Powerful (optional 4: difficult
or long synthesis))
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
5: I help you.
(Inside joke for creativepyro)
I never asked for this.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CrEaTiVePyroScience  | | (P.S: With exotic I mean : 1. Not much information to find of on google 2. (Almost) none youtube videos about it. 3: Powerful (optional 4: difficult
or long synthesis)) |
Hmmm, plutonium(IV) perchlorate looks interesting, I have found no videos on youtube, it has a lot oxygen and looks as a powerful oxidizer and I think
it would be a pretty good project to make a few grams for a rocket.
You have mentioned a few things, let's see.
Ammonium-nitrate: hygroscopic, they don't use it in pyrotechnics.
Ammonium-chlorate: unstable stuff, if it dries out it may explode.
Ammonium-perchlorate: used in a lot pyro stuff.
Aluminium nitrate: hygroscopic, it has 9 molecule water in it.
Aluminium chlorate: also highly hygroscopic, it crytsallizes as nonahydrate.
Aluminium-perchlorate: the same as above: it is usually as a form of nonahydrate.
Potassium compounds are good, they are used.
Sodium compounds are also highly hygroscopic.
Gallium nitrate is also aviable as a nonahydrate and it would cost a lot.
Gallium chlorate has not nuch info, but I think it is also a hygroscopic stuff...
Tin, magnesium, zinc, they are all hygroscopic salts, Mg-perchlorate is a commonly used dessiccant in labs.
So. Use google, use Gmelin and think a bit.
I would suggest periodates, bromates as potancial "exotic" oxidizers, potassium-iodate works well, and any other alkali compounds would be also fine.
Something what is a bit "extreme" /not an oxidizer, just an interesting inorganic chemical, espercially if you want to use it in pyrotechnics /: /:
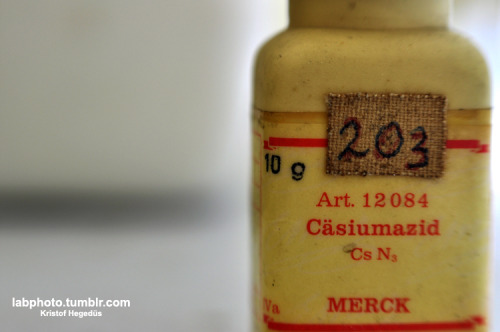
P.S.: you wrote "Will make soon a list on how to make all those exotic oxidizers." -this is basic inorganic chemistry, most forum members know these
tricks(:
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
What about perborates? Are they strong oxidizers?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
This is my compound! I made a thread about it and no one posted anything, seriously I have made like 5g of sodium perborate now, I could test it for
pyrotechnics purpose even if I dislike pyrotechnics and it's not my intended purpose.
I don't think it would be good tough, it is an adduct of metaborate and hydrogen peroxide...
[Edited on 18-7-2012 by plante1999]
I never asked for this.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Perborates are weak oxidisers. I have made a few grams and it wasn't so effective as I wanted. It good for orgo chemistry, but not for inorganic pyro
stuff.
Also: a lot solid is produces after it is "burned", so it is not an ideal fuel for pyrotechnics.
[Edited on 18-7-2012 by kristofvagyok]
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Can potassium dinitramide be used as an oxidizer? I don't think it would be very good.
How about explosive KO3? I imagine you could make it if you have an ozone generator and some potassium (from Mg and KOH, see the sticky thread). I
don't think it is very wise to actually make it, though. It is dangerous enough when pure.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Everything has a purpose, for most pyrotechnics the objective is safety, predictability, low price. Or for flashes-a lot of gases and hot particles,
fast. Or for propellants-high volume of given products out of low initial volume. For some propellants it's good adding heavy metals to the fuel,
where the engine is volume restricted and way smaller the the rest of the rocket. The limiting factor for termites is the boiling points. Lead nitrate
isn't too exotic but should be reactive and dense.
|
|
|
DoctorOfPhilosophy
Hazard to Others
  
Posts: 130
Registered: 12-6-2012
Location: Ontario, Canada
Member Is Offline
Mood: enthralled
|
|
If you want a really exotic oxidizer, xenon tetroxide should do the trick. Just keep it under -40 while oxidizing.
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
@kristofvagyok Thanks that helped.
Seems intreseting but I do have to point out that Ammonium nitrate is used in pyrotechncis, as ANFO (Ammonium Nitrate / Fuel Oil) very famous
explosive.
Do you think I can get dry Zinc , aluminium,magnesium & tin (nitrate/chlorate)when I put it for several days in a dessicator NaOH bag? Once it's
removed from the bag it will be mixed directly when a reductor and tested would it work? Not planning to store exotic oxidizers just to use them in a
short time.
About the gallium nitrate it costs me about 2euro a gram which is expensive of course but not too expensive, any oxidizer which costs les then
10euro/gram to make is okay for me. And it is indeed hygroscopic but you can get a very dry powder with the NaOH dessicator trick, gallium nitrate is
also rarely used in medics.
@Plante1999 Haha true, thanks mate ;D
@DoctorOfPhilosophy Seems hard to make and It wont be easy keeping it at -40°C and will require alot of liquid nitrogen. And its classified as an
explosive :/ but thanks for thinking.
@Rest: open for more ideas to expand my list & thanks everyone for participating in this discusion. And remember I am not searching for (exotic)
explosives but for oxidizers!
[Edited on 19-7-2012 by CrEaTiVePyroScience]
[Edited on 19-7-2012 by CrEaTiVePyroScience]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Dinitramides (K<sup>+</sup>, NH4<sup>+</sup> are perfect
oxidizers, you should try them. are perfect
oxidizers, you should try them.
Peroxodicarbonates are weak oxidizers made by electrolysis of carbonate solutions at -20°C.
Zinc peroxide.
Liquid NO2.
Rest In Pieces!
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Nitrogen trifluoride is a powerful oxidant... Might be hard to use also.
People have tested a lot of oxidants for pyro, and very few work well. If you read the Conkling you will see that the types of oxidants that work
are a small set, due to hygroscopic nature, instability or lack of oxidative ability. Here is a bit of his list of requirements:
1. The oxidizer must be quite low in hygroscopicity, or the
tendency to acquire moisture from the atmosphere. Water
can cause a variety of problems in pyrotechnic mixtures,
and materials that readily pick up water may not be used.
Sodium compounds in general are quite hygroscopic (e.g.,
sodium nitrate - NaNO 3 ) and thus they are rarely employed.
Potassium salts tend to be much better, and are
commonly used in pyrotechnics. Hygroscopicity tends to
parallel water solubility, and solubility data can be used
to anticipate possible moisture-attracting problems. The
water solubility of the common oxidizers can be found in
Table 3.2. However, it should be mentioned that large
quantities of sodium nitrate are used by the military in
combination with magnesium metal for white light production.
Here, strict humidity control is required throughout
the manufacturing process to avoid moisture uptake,
and the finished items must be sealed to prevent water
from being picked up during storage.
2. The oxidizer's positive ion (cation) must not adversely affect
the desired flame color. Sodium, for example, is an
intense emitter of yellow light, and its presence can ruin
attempts to generate red, green, and blue flames.
3. The alkali metals (Li, Na, K) and alkaline earth metals
(Ca, Sr, and Ba) are preferred for the positive ion.
These species are poor electron acceptors (and conversely,
the metals are good electron donors), and they
will not react with active metal fuels such as Mg and Al.
If easily reducible metal ions such as lead (Pb +2 ) and
copper (Cu +2 ) are present in oxidizers, there is a strong
possibility that a reaction such as
Cu(N03 ) 2 + Mg -> Cu + Mg(NO 3 ) 2
will occur, especially under moist conditions. The pyrotechnic
performance will be greatly diminished, and spontaneous
ignition might occur.
4. The compound must have an acceptable heat of decomposition.
A value that is too exothermic will produce explosive
or highly sensitive mixtures, while a value that is
too endothermic will cause ignition difficulties as well as
poor propagation of burning.
5. The compound should have as high an active oxygen content
as possible. Light cations (Na+,
K+, NH,, + ) are desirable
while heavy cations (Pb +2 , Ba +2 ) should be avoided
if possible. Oxygen-rich anions, of course, are preferred.
6. Finally, all materials used in high-energy compositions
should be low in toxicity, and yield low-toxicity reaction
products.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Just sayin about point 5. We better look at oxygen content on volume basis, Ba(NO3)2 has much more oxygen volume based then KNO3. Correct me if I'm
wrong but my Ba(NO3)2 flash is maybe faster then my KClO4. The high density also provides inertial confinement, so depending on intended usage, lead,
barium nitrate may be the choice.
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
UPDATE: Magnesium nitrate is indeed hygroscopic like mentioned before but when stored in a NaOH dessicator and used within 30minutes once removed
from dessicator , it's usefull in pyrotechnics. Although it won't have any commercial use I did achieve some results very similar to it's potassium
compound. A fast deflagration and very noticable white (slightly greenish) flash was visible when mixed in stiochmetric amounts with zinc powder
(900mesh). Since zinc isn't even that reactive I wonder how it will perform with aluminium powder. The footage will soon be uploaded on my youtube
channel.
As for the others, I've also tested lead which gave some intreseting results , burning even faster but it's hard to see the flame when it deflagrates
since the flame is grey but you can hear a popping sound.
As for the gallium nitrate things are looking less promising , but I will run some more tests tomorrow.
|
|
|