| Pages:
1
2 |
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
sprengel explosives
Having in mind that 20ml EGDN require like 30ml wfna, 50ml H2SO4, eg, distilled water and a lot of time, the concept of detonating NO2 and RFNA is
quite tempting. A lot have been sad but I can't find any videos so here's mine
http://www.youtube.com/watch?v=0JJcFOODDnk&feature=youtu...
I use dnt/tnt for fuel so it wont be oxydised like it happened with my NA/toluene experiment. Great sound, surprising damage it's almost like EGDN.
The first fuel I can think of that wont react with the acid is NB. Then MNT/DNT probablly.
I did a 2ml scale test with NO2 and toluene in a cap from a syringe needle. It was funny, if you grab with fingers it begins to boil. It gave a decent
pop with LA indicating it's got sensitivity. The problem is that I used 400ml flask with 60% NA and copper, warm 300ml jar with salt to dehydrate the
NO2 and got only few milliliters of NO2. I have nitrite, nitrates and NA, what can I do to get decent amounts of very dry NO2?
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
I used to like the idea of mixing liquid dinitrogen tetroxide with various fuels never tried though. Electrolysis of fused nitrates should give you
some very dry nitrogen dioxide. Potassium nitrate melts easy at less than 400 degrees, calcium nitrate might generate NO2 from destructive
distillation.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
So far quite yielding method for me is dropping the most useless coins in 60% nitric. But then I have to dehydrate it and I don't know why the last
time I was left with so little product. Interestingly the salt smelled of chlorine after the reaction. Do you think I can use ordinary steel and
aluminium tubes or it will contaminate the product and give even more unstable mixtures?
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
Use the birkland-eyed electric arc process. Instead of reacting the oxides of nitrogen with water, just condense them in a very cold jar.
First, dry the air, then run it through an electric arc. After the air passes through the arc, it will contain a small amount of nitrogen monoxide and
nitrogen dioxide. The part that I think might be a problem is : will air that contains very small amounts of nitrogen dioxide deposit it on a very
cold surface.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Hey man, I'm on a budget here. The 60% nitric costs like cents... I got rid of my old setup. Now I took 500ml flask. I took nice silicon hose, and
nice inline setup for dehydrating. I putted technical nitric and stuffed old coins and pieces of copper. At first the reaction was very vigorous, then
it kinda slowed and didn't get fast as it was even when I headed it and added my fuming nitric, I could still feel some of the copper is undissolved.
It's like the copper nitrate prevents further reaction (trough I doubt that). Other problem is that the reaction gives too much NO/N2O3. My silicon
hose was filled with condensed green cr*p  I even added oxygen injection. But I
was still stuck with N2O3 already in my product. It seems that O2 doesn't oxidize N2O3 in liquid phase. Now I again have about 1ml of NO2 I even added oxygen injection. But I
was still stuck with N2O3 already in my product. It seems that O2 doesn't oxidize N2O3 in liquid phase. Now I again have about 1ml of NO2  /maybe 2ml, the second ml in my blood. Now I consider nitrite and HCl injection and
heavy oxygen enrichment. /maybe 2ml, the second ml in my blood. Now I consider nitrite and HCl injection and
heavy oxygen enrichment.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Glad to see somebody is finally trying it out. I'm jealous of your cheap nitric!
I haven't put a whole lot of research in to HNO3 --> NO2 options becuase HNO3 was never plentiful for me, only nitrate salts.
There are already several threads about this
http://www.sciencemadness.org/talk/viewthread.php?tid=134#pi...
http://www.sciencemadness.org/talk/viewthread.php?tid=14169
I really like this novel way:
http://www.sciencemadness.org/talk/viewthread.php?tid=12966#...
Even adding a catalytic amount of Mn to molten K(Na)NO3 may work well to yield a NO2+N2O3 mix.
A good place to learn about molten nitrate decomposition: http://books.google.com/books?id=NzhUTvUkpDQC&pg=PA624
I know that pyrolysis of xCa(NO3)2*xNH4NO3*xH2O does have a good yield of NO2 mixed with O2 and small amounts of H2O, etc. Steel container on a
propane burner works pretty well, but you do have to be careful the outlet tube doesn't get plugged.
For smaller amounts of more pure NO2, I'd say using a nitrite with an acid and injecting pure oxygen (cutting torch tank) to fully oxidize the N2O3 is
your best bet. Most people talk about NaNO2 + conc. HCl, but I'd say NaNO2 + NaHSO4 would be better and yield fairly dry, pure N2O3. Then mixing with
the correct amount of O2 will get you clean NO2. The reaction of O2 with N2O3 can take a minute or two, so having a buffer volume (series of large
glass jars, 5 gal bucket, etc) plumbed in before the condenser will allow a complete reaction.
If you use a method that yields NO2 without O2/N2 contamination, it should be easy to condense with salt+ice bath. If you make NO2 with O2/N2 in it,
you may lose lots even with a CaCl2+ice bath. A solid CO2+acetone bath would be the way to go in that case if you can get it cheaply.
Sadly the Birkeland-Eyde process usually only makes <3-5% NO2 from air. Simply cooling that won't work. If there was another way to capture the
NO2, it could be very attractive. Maybe bubbling through a cold solvent? Could be worth playing with, especially as nitrites/nitrates gradually become
harder to get.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
So there were the topics of NO2 hiding. I had a decent method of making NO2 when I was a kid. I mixed KNO3 and CuSO4 solutions, boiled them down and
finally collected the NO2. It took me a while before I began to question is it pure NO2 or just some dilute nitric acid with dark color. I didn't know
about any explosives back then, but still I was very exited and was sure it can form strong explosive mixtures. I just tried the nitrite process. I
wasted 40ml HCl and quite a lot of nitrite and peroxide. Record yield, 5ml  You
say it takes a while till the N2O3 reacts with O2, may be that was a little problem. Also how can N2 and O2 impurities keep the NO2 from condensating? You
say it takes a while till the N2O3 reacts with O2, may be that was a little problem. Also how can N2 and O2 impurities keep the NO2 from condensating?
Anyway NO2 is supposedly more chemically inert then HNO3, doesn't require so chemically resistant container, the fuel doesn't need to be so resistant
to oxidation and if you're lucky you'll be able to produce it from nitrate salt without H2SO4, distillations and stuff. The drawback is the narrow
range between boiling and melting and things get even more interesting with N2O3 contamination.
HNO3 on the other hand is guaranteed to be liquid under wide temperature conditions, dissolves nitro aromatics, nitromethane and rdx  . If it almost doesn't react with toluene I wonder if it really wouldn't react with
benzene. As it's considered the more reactive option, you don't have to worry about NOx contaminents, as they'll make it only more inert IMO Along
with the higher reactivity, if you get some moisture in the HNO3 the fuel may separate. . If it almost doesn't react with toluene I wonder if it really wouldn't react with
benzene. As it's considered the more reactive option, you don't have to worry about NOx contaminents, as they'll make it only more inert IMO Along
with the higher reactivity, if you get some moisture in the HNO3 the fuel may separate.
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
What about just a simple liquid NO2 and charcoal explosive? Maybe just as good as liquid oxygen and charcoal?
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Nah, H2O2/aluminium would beat it at every parameter. But HNO3/charcoal would probably be the safest HNO3 binary.
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
I think most liquid oxygen explosives do not need a detonator, they easily explode from fire. Do the liquid NO2/fuel explosives behave the same way?
would HNO3/charcoal need a detonator?
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I think NO2 (which is actually N2O4 in liquid state) with a stoiciometric amount of liquid fuel would detonate from flame, possibly confinement would
be required. The sensitivity can be adjusted over a wide range by varying the stoiciometric ratio. There is a patent that talks about that, using
kerosene, more than 10-20% off stoiciometric and it became quite insensitive to impact. Haven't seen much test data for flame/spark sensitivity, so
its all speculation really...
N2O4/HNO3 with charcoal would probably be quite insensitive, maybe to the point of requiring a large booster. Adding charcoal (or something else
powdered) to a N2O4 rich mix with hydrocarbons might be a way to achieve acceptable sensitivity while retaining maximum power...
I'm pretty sure liquid oxygen mixtures are always going to be way more sensitive. Just look at how many things it ignites on contact with...
EDIT
If you subject HNO3 to electrolysis with certain electrodes (carbon, etc) in a divided cell, one side will release NO and the other O2, if you just
dry and mix those and provide enough buffer space to allow full oxidation of the NO, you could get very clean NO2 efficiently, and without using high
temperatures or reagents other than dilute HNO3.
They also say if you bubble the NO directly in to the opposite half of the cell, it will be reoxidized back to HNO3 and you can concentrate 70% up to
99% HNO3... Why hasn't anyone tried that?
If you use different electrodes/conditions in the cell it will reduce all the way to hydroxylamine, and then ammonia.
Maybe even more interesting, if you bubble ethylene in to a solution of Ca(NO3)2 in acetone under electrolysis, EGDN is produced directly... It does
require a divided cell, but if they can pull it off in 1938, it can't be so tough today!
You could also be producing ethylene simultaneously in another cell by electrolysis of acetate.
So much fun to be had!
The sources: http://www.sciencemadness.org/talk/viewthread.php?tid=1716
Edit #2
Shannon, I don't think its a that simple unfortunately... Pretty sure N2O4 and high %HNO3 are miscible or very soluble in each other... Also oxygen is
required for N2O4 + H2O reaction to approach completion. And as HNO3% rises it can actually reverse under certain conditions. If you or anyone else
really want to understand these dynamics download the old free edook "The Absorption of Nitrous Gasses" it is a great read, covers almost every aspect
of HNO3 manufaction and even includes enough detail to be useful.
Not to say that liquid N2O4 can't be used to help make >azeotropic HNO3, its just not quite that easy/simple.
[Edited on 29-7-2012 by 497]
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
I have read that mixing liquid NO2 (N2O4) with diluted HNO3., then vigorously mixing, then let it settle, two layers will form, one of those layers
will be 99% HNO3. I have tried desperately to find this book again.
Anybody ever heard of this?
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
I did not think it was that simple either. I could not believe what I was reading, but I can assure you, that is what it said. I searched for years to
find a way to concentrate nitric acid without using sulphuric acid. I really like the calcium nitrate method, all you need is dilute HNO3 and oyster
shells.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
There not so many things out of witch conc. HNO3 can separate as layer. Can't you just distill H2SO4/nitrate salt? It works great and the acid you
get can even be used for rdx.
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
Yes I have made nitric acid from H2SO4 and potassium nitrate, but I want a method that only uses natural materials from the land and not store bought
chemicals. Nitrates from animal matter. The problem is sulphur is not found very much where I live, so I cannot make sulphuric acid from the land.
Any method to make nitric acid that does not need sulphuric acid, I get very excited! Just IMO.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I didn't know one can get fixed nitrogen without catalitic oxidation of ammonia or high temperature generation of NOx. You can decompose your nitrate,
get NOx and end up with conc. HNO3 after series of distillations.
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
The big question is just how concentrated of acid can be made from NOx and water WITHOUT using sulphuric acid. 68%? 95%?
I get different answers from different sources, who is right?
I was going to try the biochemical formation of calcium nitrate, then heat it to drive off NOx. This would be an almost all natural, no bought
chemical method of making HNO3. I am close to giving up this idea, as it seems impossible.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
The folowing is a " what if " excercise. I have not personally prepared any of the
materials discussed below here , and do not make any representation other than
what I explicitly have stated. For practical investigation take necessary precautions
to prevent breathing NO2 which will destroy your lungs , safeguard your eyesight
and fingers against unexpected detonation , be wary if hydrogen cyanide is evolved ,
breathing it even slightly will kill you.
N2O4 has a density of 1.44gm/cc providing a means of achieving liquid explosives
of high density. Organic fuels only have densities that cluster around that of water
1gm/cc. To achieve a high density mixture , a fuel such as Potassium Ferricyanide
with density of 1.89gm/cc can be used providing the two are miscible as a solution.
Wether this may react to form the nitroprusside I don't know.
The issue of safety arises since this will have a sensitivity of a primary explosive ,
for this reason suitable only for on site preparation.
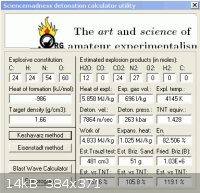
4 K3Fe(CN)6 + 15 N2O4 => 6 K2O + 2 Fe2O3 + 24 CO2 + 27 N2
22 parts by weight of ferricyanide to 23 Parts dinitrogen tetroxide ( almost exact )
Multiplying the density of each part by it's weight fraction in the mixture :
(1.44 X 23/45 = .736) + (1.89 X 22/45 = .924) = 1.66gm/cc , for the blend.
Of course density in solution will be less than as crystaline so it will be less.
Heats of formation are : - 19.5 kJ/mol for N2O4
- 173.5 kJ/mol for the ferricyanide.
The total for the mixture amounts to - 986.5 kJ
Ab initio methods of estimating performance of C H N O explosives do not account
for metal composition. As such , using the utility devised by forum member Engager
to make an assessment , hydrogen was entered in place of Potassium and Iron
at an equal bond count. The energy derived falls short by 916 kJ and gas results
must be discarded. The estimate of 7800 m/s detonation rate , 260 kbar , and
1.4 X T.N.T , seems reasonable.
______________________________________________
As discussed in U.S. patent , 2355817
mixtures with conventional nitro compounds reduces the proportion of N2O4
required , overall the density is greater than with un-nitrated organics.
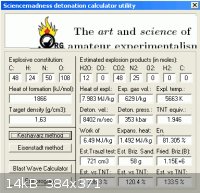
8 C2H3N3O7 + 13 N2O4 => 48 CO2 + 12 H2O + 25 N2
3 parts by weight of Trinitrophenol to 2 Parts dinitrogen tetroxide ( almost exact )
Trinitrophenol has a practical density of 1.66gm/cc , it's crystal density of
1.76gm/cc is used to estimate in solution with N2O4 by multiplying the
density of each part by it's weight fraction in the mixture :
(1.76 X 3/5 = 1.056) + (1.44 X 2/5 = .576) = 1.63gm/cc , for the blend.
as noted density in solution will be less than as crystaline so it will be less.
Heats of formation are : + 265 kJ/mol for picric acid ( Urbanski )
- 19.5 kJ/mol for N2O4
The total for the mixture amounts to + 1866.5 kJ
The performance estmated by the utility shows substantial improvement
beyond that with ferricyanide or of picric acid alone.
______________________________________________
Same as above U.S. patent , 2355817 and as discussed in U.S. patent 5140908
A solution of trinitrophenol in nitromethane can be augmented by a small addition
of N2O4 which is technically easier to prepare and allows conservation of nitromethane.
It also allows the proportion of picric acid to be 80 %
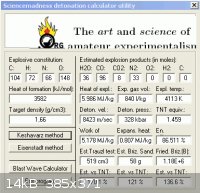
16 C6H3N3O7 + 5 N2O4 + 8 CH3NO2 => 8 CO2 + 96 CO + 36 H2O + 33 N2
TNP - 79.44 % , N2O4 - 9.97 % , NM - 10.38 % , or approximately
8 parts by wegiht of Trinitrophenol to 1 part nitromethane and 1 part N2O4
The density of nitromethane is 1.13gm/cc , multiplying the density of each
part by it's weight fraction in the mixture :
(1.76 X 8/10 = 1.408) + (1.44 X 1/10 = .144) + (1.13 X 1/10 = .113) = 1.66gm/cc
again density in solution will be less than as crystaline so it will be less.
Heats of formation are : + 265 kJ/mol for picric acid ( Urbanski )
- 19.5 kJ/mol for N2O4
- 70 kJ/mol for nitromethane
Total for the mixture amounts to + 3582.5 kJ
The performance estmated by the utility shows comparable performance
to that of a stoichiometric blend of picric acid with N2O4 alone , which
demonstrates the value of admixtures with N2O4 over others. It is also
the most economical in the use of the more expensive ingredients.
[Edited on 30-7-2012 by franklyn]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I agree, finding higher density fuels would be very important to maximize the liquid binary's performance.
http://www.sciencedirect.com/science/article/pii/S0020169300...
I think dry ferricyanide+N2O4 probably wouldn't react.. Solubility is another thing though. I'd be amazed if it was that soluble.
http://144.206.159.178/ft/986/64152/1092784.pdf
PolyVinylpyrolidone forms a stable solid 1:1 complex with N2O4 (and HNO3), maybe useful as a fuel in a mixture with further oxidant? Composition of
N2O4 complex is (C6H9N3O5)n.
http://proj3.sinica.edu.tw/~chem/servxx6/files/paper_3091_12...
Better yet, polyethylene glycol forms a stable complex with N2O4. After boiling off the solvent and drying in a vacuum, there was still a 40% weight
gain. Then they say "it was determined the capacity of the polymer was 2.5 mmol N2O4 per g" which would correspond to a 32% weight gain, so its a
little confusing... Their diagram of the complex is very misleading too, makes it look like you could actually get a (C2H4O*N2O4)n, I wish... If you
saturated it in N2O4 and didn't vacuum it off, you could reach around 100% weight gain and (C2H4O*NO2)n at best... According to various sources ethers
bind to N2O4 more strongly than other solvents besides amines/amides. It could be probably be used as a gelling agent/stabilizer for mixtures of
solids+liquids that would normally separate, maybe even allow a moldable plastic like material?
http://www.imm.ac.cn/journal/ccl/1202/120202-99-20364-p4.pdf
Dioxane N2O4 complex is stable liquid, C4H8NO4. As with the polyglycol, the N2O4 is bound strongly enough that the complex is a weak oxidizer. Weak
enough to selectively turn oximes to ketones in the presence of many other oxidizable functional groups. This paper also has a nice layout of how they
produced clean dry NO2 gas from NaNO2+H2SO4+O2.
http://pubs.acs.org/doi/abs/10.1021/bk-1996-0623.ch004
This is really cool. Basically you can make your own nitroarenes in situ with O2 as the oxidant... If one was going to the effort to make liquid N2O4,
it would be great to be able to create powerful nitroarene mixtures without messing around with a whole separate nitration reaction to make said
nitroarenes. Keeping in mind HNO3 is produced as a byproduct...
Another possible fuel, acetylacetonate salts are soluble and stable (at least for a matter of hours) in N2O4. Used as catalysts for aromatic nitration
with N2O4.
Finding plentiful and convenient things to react directly with N2O4 is also a worthy pursuit. I looked in to it a long time ago, but I've forgotten a
lot of that by now, and I'm wayyy too tired to go wading thorough hundreds of pdf files looking for them... Having a far less harsh complexed N2O4
reagent undoubtedly opens up a lot more possibilities too!
If you already have a polymer+N2O4, could reasonable VOD be maintained, density improved by supplementing it with a powdered solid oxidizer? Maybe one
of those nice anhydrous transition metal nitrates that are so easy to make with N2O4?
| Quote: |
The big question is just how concentrated of acid can be made from NOx and water WITHOUT using sulphuric acid. 68%? 95%?
|
It's not so much of a "limit" to the concentration possible from NOx+O2+H2O as a gradual drop in reaction rate and efficiency as the %HNO3 rises. If I
remember correctly 50% can be achieved with relative ease, as you go over 70-80% it starts to become impractically slow/inefficient. The concentration
of NO2+O2 in the gas also makes a big difference with this... If you have a straight NO2/O2 mix it could reach over 90% eventually. It's been a long
time since I read about this stuff.
Magnesium nitrate is well known to break the H2O+HNO3 azeotrope, there is really no need to take the acid above %50 with NOx. Calcium nitrate may work
as well. Google it, there's no shortage of information on the subject.
If you just want NOx to make HNO3 by reacting with water, why mess around with organic sources of N in complex mixtures when you can directly fix it
from the air with no reagents? It's not super efficient, but works reliably with electricity+air+water. And compared with pyrolysis of Ca(NO3)2, it
will be more convenient, trust me. I've tried both ways. While an arc won't make tons of NO2 per hour, it can run hands off day and night, and there
is no risk of suddenly spewing molten nitrates (which will ignite organics on contact btw) or a large cloud of concentrated NO2...
In fact, the more 21st century solution to this problem would be something like this: http://pubs.rsc.org/en/content/articlelanding/2009/ee/b90647...
Polyethylene glycol is not hard stuff to come by at all. Brake fluid is largely tri and tetraethylene glycol alkyl ethers with a smaller fraction of
>20 long polyethylene glycols. No doubt it would work. Or you can get any length you want in the range from 2 to 10000 units long online as
ingredients for making cosmetics, etc. Ethylene oxide is cheap to make these days, so it's polymers tend to be also. Usually not more than $10-20/lb
for smaller amounts. No doubt there are OTC products it could be isolated from too.
Absorb/flow it on to a porous media of your choice and pipe in the NO2. It will provide a few key advantages over water: much faster and more
efficient absorption in a smaller space, no need for reoxidation of the HNO2 produced by NO2 reacting with water (N2O4 + H2O --> HNO3 + HNO2), easy
thermal regeneration that would provide you with high purity N2O4 that is easy to condense or use in a reaction. Conveniently it should have a
relatively sudden "saturation" of the polyethylene glycol so you can easy tell when it needs regenerating. Another added bonus, it would allow you to
run your arc much reactor more efficiently. The kwh/kg NO2 increases nearly exponentially as you produce higher NO2% in air, so if you could
conveniently produce under 1% NO2 and not have the problems that arise with trying to absorb that in water it would save you megawatts over time.
Efficiency of absorption into water really starts to drop off below 2-3% NO2, yet you sacrifice a lot of arc reactor efficiency to produce over
3-4%... Those two conflicting optimums of the arc reaction and the water absorption tower (along with harder scale up vs ammonia oxidation) are
basically what have prevented the Birkeland-Eyde process from becoming widely used. Speaking of which, maybe I should continue this side of the
discussion over in that thread..
According to the above abstract, the PEG 50 on mol sieve is able to absorb about 25-30% of its capacity (based on the above paper where they reacted
it with pure NO2) before letting much NO2 escape. I would guess that if you used higher concentration NO2 (they used .2%), slower and colder gas
flow, it could be increased above 50-70% of theoretical.
That means if you had a column with 1kg PEG in it you'd have to regenerate it every time you got between 50-150g NO2 absorbed... Not bad. If you
wanted even lower maintenance for a larger scale model, a continuous loop of liquid PEG could be circulated into a separate regenerator where waste
heat from the arc reactor could boil the NO2 out of it. Use an "airlift" style pump to allow the boiling NO2 to provide the lift to bring the PEG back
to the top of the absorber...
I have hope that as humanity encounters inevitable adversity/instability, the (perceived) value of decentralization and localization of all resources
will increase... Intercontinental transportation of our entire resource supply is simply not robust enough to remain cost effective forever! It is
becoming obvious that economies of scale won't solve all our problems. So I'd like to get a jump on all that and start figuring out how to scale all
this "industry" back down to a reasonable size. It may seem like a tall order for certain situations, but how hard has anyone even tried in the last
few decades?
[Edited on 30-7-2012 by 497]
[Edited on 30-7-2012 by 497]
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
What's wrong with intercontinental transport of stuff? So if someone does not live near a copper mine they should not have copper wiring in there
house?
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
What dot you think about a sprengel explosive that uses two oxidizers and two fuels, two powders and two liquids. N2O4 and kerosene absorbed into
calcium nitrate and charcoal. It could be adjusted so that 10% of the oxygen comes from N2O4 and 90% comes from calcium nitrate, this would keep it
from being so sensitive. Or another way to describe it is a very sensitive (N2O4 /kerosene) absorbed on a not very sensitive (calcium nitrate
/charcoal) . This similar to what somebody already said few post back, but I add a powder oxidizer too.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
If you have calcium nitrate based explosive over 80%, then you wont save much if you replace the classic ng with NO2/fuel. I think the value here is
that with a high voltage transformer at home and a suitable fuel you can make C4-like stuff. I prefer talking about cheap, low tech fuels.
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
I very much agree, a high voltage transformer and instant high explosives that will out perform some commercial and military explosives! ! And a
detonator not necessary! Life is good!
Calcium nitrate should be easy using birkland-eyed, just bubble the air/NO2 into water with oyster shells. Calcium nitrate is very useful, use it to
make N2O4, or nitric acid, to concentrate nitric acid (break azeotrope with water) or as a oxidizer in various explosives. If you heat the calcium
nitrate to make NO2, use the leftover calcium oxide to make glycerine from Bacon grease. High explosive from only electric arc, oyster shells and
Bacon grease.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Putting any of your dangerous hare-brained 'schemes' into effect can change all that in microseconds . . .
|
|
|
shannon dove
Hazard to Self
 
Posts: 77
Registered: 30-11-2011
Member Is Offline
Mood: No Mood
|
|
Of all the stuff talked about on this forum, why are my ideas dangerous?
Alright then, I will just work with safe and stable chemicals like ethyl perchlorate, and nitrogen trichloride and binaries like white phosphorus and
potassium chlorate.
|
|
|
| Pages:
1
2 |