| Pages:
1
..
10
11
12
13 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>halogen</strong>, <a href="viewthread.php?tid=15455&page=28#pid236897">that paper</a> is already in
<strong><a href="forumdisplay.php?fid=21">References</a></strong>. This has also previously been discussed for Na, K, and Li
in several threads, if I recall correctly.
[Edited on 25.9.13 by bfesser]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Ah, thanks. So they cheated using sacrificial alkali anodes.
Though, interestingly as the AlX3 adduct seemed to produce an alkali deposit, maybe some kind of aluminum anode or zinc would suit the amateur.
Nonetheless I'm curious what might happen were there an inert anode. For what it's worth electrolysis of organics in HF is a known flurination, it
might be interesting to examine the results of electrolysis of fluoride salts in non-aq. solution.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
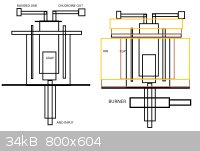
I am planning to reutilize my micro-castner soon and post some pictures because I'm gonna retry the chlor-alkali - combination process with molten
NaCl:CaCl 70:30 at 650C. The reactor design is following (and later you see picture of the reactor I have constructed earlier at springtime) -
ATTACHMENT. The reactor essentially consists of anode from underside and cathode around the reactor from upside, and I will contain it within clay an
layers of rockwool to make it a lot more energy efficient. I'ts made out of 316 steel and I'll be using graphite anode and iron cathode with 5V at
about 50-100A depending on the conductor's cross diameter and EC values. This is rather low power, but since it could procude sodium and clean
chlorine gas for chlorination processes, I'll give it a go-code. The chlor-alkali will be waiting for polymer membrane or 2 kilograms of mercury until
procceeding.
The reactor volume is about 4 liters so it should ideally hold about 6-7kg of chloride salts, which of about 5kg is sodium based and will be
electrolyzed, and of this, in theory, about 2000 grams of sodium and 3000 grams of chlorine gas can be obtained. Heating should be carried out using
6kW propane torch.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I made test run with my electric heater. This is molten sodium chloride from my reactor, 500 grams, the color is from temperature.

|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
testimento, maybe I'm misunderstanding the diagram.
Are you collecting sodium metal at the top of the cell, with the
molten metal in contact with the cathode? If so, this may give
you some trouble.
The old literature generally shows the cathode on the bottom of
the cell, with the sodium metal collected as it floats to the top.
I used a fireclay ceramic as a divider once, thinking that it
would allow me to collect sodium on one side, and chlorine on
the other; with the electrodes coming in from the top. After a
short period of time the ceramic became conductive (@#&^!).
Apparently the salt electrolyzed through the ceramic,
contaminating the melt in the process.
It takes a high-quality ceramic to work under those types of
conditions.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
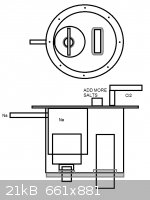
I was thinking this type:
http://images.flatworldknowledge.com/averillfwk/averillfwk-f...
The anode shall be put through the bottom with plaster fitting that insulates it from the bottom, and the cathode through the top flange that is
lifted off when the system is opened.
How the sodium shall interfere with the cathode? What it does? Should I consider this structure straight away?
https://s3.amazonaws.com/readers/2010/02/17/downscellschemat...
[Edited on 28-10-2013 by testimento]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
In normal cell operation, the diaphragm (and collected sodium)
potential is partway between the anode and cathode potentials.
When the cell is operated at the correct current density (i.e.,
not too high of a cell potential), the metal diaphragm remains
electrically inert. If the cathode comes in through the top of
the melt, then the sodium can collect around it, shorting the
cathode to the diaphragm. At this point the diaphragm
becomes the cathode, and sodium is produced in the same
place as the chlorine.
If a ceramic insulator is used under the same conditions, with
the intention of isolating the sodium metal from the diaphragm,
care has to be taken in the selection of its material. The lousy
fireclay separator that I used was apparently not dense/inert
enough, and the sodium electrolyzed right through it, essentially
making it part of the cathode. Sodium beta-alumina is
discussed as a diaphragm material in at least one thread here
(maybe this one), and may work under these conditions.
The image that you linked to actually shows the cathode and
sodium metal electrically isolated from each other. That might
not be obvious right away.
If you want to run the cathode in through the top, you can see if
it's possible to electrolyze the sodium metal into a solid alloy at
the cell temperatures. That way the sodium metal is
immobilized. Na-Pb or Na-Sn systems might be a place to
start. If you make the container itself the cathode, you may be
able to electrolyze the sodium into a porous mass of iron
powder. If that worked current density would be critical, in
order to keep the sodium from just forming on the surface
closest to the anode. It'll probably be easier just to do it the
conventional way, though (with the cathode entering through
the bottom, or the side).
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I have understood that no diaphragm is needed for this operation, as long as the electrodes are accordingly separated. I've redesigned the system to
prevent the cathode from touching the sodium formed, and the metal is expected to accumulate on the top, and flow out of the reactor when the
container is full and more sodium chloride is to be added.
Btw, as a quick off-topic, are there effective ways to make chlorine gas into hydrochloric acid?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
If you're not worried too much about efficiency, the diaphragm
is not needed. What it allows mostly:
1. The cathode and anode can be very close together without
the products mixing. The geometry of the cells is designed
mostly to allow maximum current and efficiency, and minimal
use of supplemental heating or cooling. Generally this means
that the electrodes have to be pretty close together, and the
current density has to be consistent across the electrodes. For
your purposes you can run a lower current density and use
supplemental heating, so the electrode spacing is not as
important.
2. It helps circulation of the electrolyte. The diaphragm is
porous enough to allow the molten salt to circulate through it,
without allowing the chlorine or sodium to mix. Again, if your
current density is low, and your heat comes from supplemental
sources, this is not so important. If you're using solid metal
containers around your cathode to catch the sodium as it floats
off, you have to be careful not to run the current up so high that
the resulting turbulence carries your sodium right back out and
into the chlorine compartment (or vice versa).
You can try this with regular water electrolysis. Even with both
electrodes in inverted test tubes, at high currents the turbulence
will carry the gas bubbles right out the bottom.
I think that other people can answer the Chlorine to HCl
conversion question better than I can.
[Edited on 28-10-2013 by WGTR]
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I have my old power source rated at 400A at 6VDC, but with rewinding it can go as high as 600 amps. I was preferring a graphite electrode with CSA
500mm2 and a similar measurements for steel cathode. It's supposed to be heated up with electric heater, and I'm quite sure that the heat from the
electrolysis is not sufficient to keep it over 600C, but the temperature shouldn't be a problem.
How many millimeters do you think the distance between the electrodes should be? In my concept the distance could be limited to ~100mm minimum, but
changing the design to square instead of spherical, it could be lowered to maybe 10mm minimum. The schematics show that commercial cells use only
steel mesh to prevent the chlorine and sodium mixing. I'd rather drive the cell with more power(to a sensible extent, of course) than make the
structure more complicated, because in amateur setups, the simplicity usually inhibits costs and provides reliable operation. Membranes are, to my
experience, highly prone to errors.
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
You won't be able to push 400A (let alone 600A) through molten NaCl @ 6V with any reasonably-sized electrodes. If your supplemental heat can keep the
salt molten by itself then you can use just about any DC power supply. A Downs cell (particularly straight NaCl - no fancy salt mixtures) is damned
finicky. Keep in mind that your electrodes and any other 'features' will sink heat from the melt.
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I have 20 units of 10x60mm graphite electrodes, 200mm length, and I checked their conductivity is high enough for the lower part even when over 600C.
Of course, the resistance at very high temp conditions in molten salt and other stuff is not anything close from theory, but it's enough for me if I
can push 50-100 amps off that record through the pot.
A mixture of nacl:cacl 42:58 will melt at 600C. I tested this yesterday, and it's the common mixture used in downs cells. Another mixture consisting
of magnesium, barium and sodium chloride will melt as low as 450C temp, but I haven't got those at hand now.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
testimento, I can't say what your cell dimensions should
be without it being speculation, but 10mm spacing between
anode and cathode sounds too close for your type of setup.
For your NaCl/CaCl2 melt, I'd suggest solidifying some of the
molten salt on a steel rod, and trying to dissolve it in water. All
of the salt should dissolve. If some is left over, then perhaps
there is CaCO3/Ca(OH)2 contamination, and the melt needs to
be purified. The pH of the solution shouldn't be basic.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
After reading most of this topic, it's clear that Mg or Al can take oxygen from (most) group 1 metals, does this reaction seem likely to happen: 2KNO3
+ 4Al--> K + 2Al2O3 + N2?
Contrary to what some people have told me, it's easy for me to ignite a nitrate-Al mixture without sulfur.
Edit: Without waiting for a response, I went out and tried it.
I mixed KNO3 and Al in stoichiometric proportions, (as shown above). In a steel can with a brick on top, I proceeded to light it up. Soon the can was
glowing red hot, and the wood which it was on, caught on fire.
After it cooled, I saw some little balls of metal on the bottom of the can (actually almost all of the bottom of the can had melted away), I dropped a
drop of water on them, but nothing happened, I assume that they are steel nuggets. After dropping some more water on some more of the slag, I noticed
quite a bit of fizzing! It must have been potassium, the can was way to cool to boil water, and there couldn't have been any other 'active'
metals/materials in there AFAIK.
So the answer is yes, you can make potassium from KNO3 and Al, but it's very ineffective, and the product is quite impure.
I know that this topic is about sodium, and I'm sure if you switched the KNO3 with NaNO3, the result would be the same, except you would get impure
sodium, instead of impure potassium.
[Edited on 28-1-2014 by Zyklonb]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
this seems the best place to inform that a 27ish mm quartz tube, domed on one end, with a magnetic stirrer and a small 12v UV lamp set up run on two
6v lantern batteries in series, enclosed with aluminum foil reflectance, all run on a mixture of atomized copper powder and ground iodine crystals in
distilled water afforded a pure white powder, seemingly copper i iodide, CuI, which precipitated out of solution rather efficiently. settling in
layers if reccolection is correct.
prior approach using electrolysis was unsuccessful with same 2x6v series lantern batteries.
[Edited on 24-10-2014 by quantumcorespacealchemyst]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Right forum, but wrong thread.
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by testimento  | | I made test run with my electric heater. This is molten sodium chloride from my reactor, 500 grams, the color is from temperature.
|
Judging by your pic, it's ripe for electrolyzing -- maybe just a touch too hot. You're looking for a black hot, so to speak, with a touch of red glow
as seen in a dimly lit environment. If your PS puts out enough juice, you can directly brute force it by electrolysis to preheat everything then back
off to that sweet spot. Introducing additional hardware for the apparatus may tax your electric furnace beyond its capability or fail to properly fuse
the salt.
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
According to this source: http://chemiday.com/en/reaction/3-1-0-8667
sodium oxide decomposes to sodium and sodium peroxide at high heat. Also, sodium oxide can be produced by thermal decomposition of sodium carbonate at
1000 C.
Do you think that heating sodium carbonate e.g. in a tube furnace might produce some metalic Na?
Special atmosphere might be required (vacuum). Also Na evaporates at 883 C ...
Another source refering this reaction:
http://www.allreactions.com/index.php/group-1a/natrium/sodiu...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Most non-coordinating anions are hard to synthesize, but bisoxalatoborate salts can be made by simply heating oxalates with boric acid and oxalic
acid, or by heating carbonates with both acids. This patent focuses on the purification but describes the prep:
https://www.google.com/patents/US20100145076
I've been thinking of what to do about this -- lithium bisoxalatoborate is soluble in most organic solvents and has received attention as a battery
electrolyte, but sodium bisoxalatoborate is relatively unstudied. However, I believe the sodium bisoxalatoborate is soluble in solvents such as
toluene or pyridine as well. This paper considered NaBOB for a battery electrolyte before settling on the related NaDFOB (diflurooxalatoborate), and
notes that the solvent compatibilities of the oxalatoborates are better than Na perchlorate:
http://pubs.rsc.org/en/content/articlelanding/2015/cc/c5cc02...
Anyway, if you dissolve NaBOB in some sort of solvent, you can probably electrolyze this to get sodium. I'm not sure what the byproduct will be,
though. One possibility:
(C2O4)B- >> e- + B(s) + 4CO2(g)
If boron is produced at the same time as Na this would be quite a useful coincidence but I suspect the actual product may be boric acid or sodium
metaborate.
[Edited on 4-4-2017 by clearly_not_atara]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
NurdRage had this to say this morning:
<blockquote class="twitter-tweet" data-lang="en"><p lang="en" dir="ltr">HOLY @#%!$ I ACCIDENTALLY MADE BULK SODIUM METAL WITHOUT
ELECTROLYSIS. This is a MAJOR breakthrough for amateur chemistry.</p>— NurdRage (@NurdRage) <a
href="https://twitter.com/NurdRage/status/849812574854160384">April 6, 2017</a></blockquote>
<script async src="//platform.twitter.com/widgets.js" charset="utf-8"></script>
the tone at least got walked back:
<blockquote class="twitter-tweet" data-lang="en"><p lang="en" dir="ltr">hmmm... looking closely now this might not be worthy of a
peer-reviewed paper... but it's still a breakthrough for amateur chemistry.</p>— NurdRage (@NurdRage) <a
href="https://twitter.com/NurdRage/status/849817272483028996">April 6, 2017</a></blockquote>
<script async src="//platform.twitter.com/widgets.js" charset="utf-8"></script>
<blockquote class="twitter-tweet" data-lang="en"><p lang="en" dir="ltr">okay, turns out this sodium method is not novel enough for a
peer-reviewed paper. But it's useful for the amateur so i'll make a video on it</p>— NurdRage (@NurdRage) <a
href="https://twitter.com/NurdRage/status/850126265298432000">April 6, 2017</a></blockquote>
<script async src="//platform.twitter.com/widgets.js" charset="utf-8"></script>
Still, I wonder what this could be? Might be worth keeping an eye on!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
If I had to guess, using Mg to reduce NaOH, and then some means of purifying that.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Accidentally making bulk sodium... I am burning with curiosity about what he was trying to do....
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Nurdrage's latest video
confirms you are correct. But it looks like we will need to wait a bit to learn what the purification process is.
If this is as successful as it seems it could become another hime chemist standard similar to chloroform, hydrazine sulfate, distilling nitric acid,
and chlorine from TCCA.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Anyone try molten sodium magnesium oxide aggragate separation using candle wax
MgO density 3.58 g/cm³
Sodium density 0.968 g/cm³
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
is there any better way for collecting sodium in electrolysis cell than a syringe or a spoon ?
|
|
|
| Pages:
1
..
10
11
12
13 |