| Pages:
1
2 |
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
I made some nitric acid
I made some nitric acid by passing high voltage through air and mixing the NO2 into water. I also wanted to know how concentrated I could get it. I
measured the concentration at 0.93 M. The actual volume was 17.8 mL. It isn't a whole lot but it is neat making something for almost free. The only
cost is electricity.
If you like making things yourself, give this method a try.
[Edited on 6-10-2012 by vmelkon]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
What voltage, and at what current?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Congrats on a successful project.
Add some concentrated H<sub>2</sub>SO<sub>4</sub> to the dilute HNO<sub>3</sub> and distill in an all glass setup
to yield red fuming nitric acid.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
The voltage source is a 27 inch TV and they haven't printed the voltage anywhere. Thank you Sharp! Old TVs use to say 26 KV. I turn it on for 3
minutes and my large glass vessel (I think 8 L) gets all brown. I did that maybe 30 times.
Right now, I'm going to make NaNO3. I could at one point do H2SO4 + NaNO3 to get conc nitric acid.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thats really nice!
Your only reagents are the air itself 
I'm editing the message, because after some research I could answer myself some of my questions on the project. I found out the process name and it
made me find lots of valuable information on it 
However I still have a few questions. Did you heated the air before making it go through the high voltage?
Is it a cost/benefit nice method? (I mean, did you waste much electricity for making it work?)
I'm wondering because it looks like a project that takes some time and planning to work right. So its probably something to do if you are more certain
of its efficiency in case you want to use it as a Nitric Acid source.
[Edited on 12-10-2012 by IanCaio]
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
using the data provided, and assuming a TV power of 100W and $0.10/kWh, to produce 1 mol HNO3 it takes 90.6 hours of operation for a cost of $0.91.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | I also wanted to know how concentrated I could get it. |
That depends on the type of arc produced!
Higher voltage coupled with lower current will produce an arc which will produce a certain quantity of ozone in addition to
NO<sub>2</sub>!
The resulting N<sub>2</sub>O<sub>5</sub>/NO<sub>2</sub> mixture will dissolve in water to produce
HNO<sub>3</sub> much more readily than will NO<sub>2</sub> alone!
In this way, HNO<sub>3</sub>/N<sub>2</sub>O<sub>5</sub> solutions of S.G. above 1.52 may be produced.
BTW ─ any pics?
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks for the replies!
It sounds like its not much expensive then. Might be a nice project to make a small scale reactor 
I was looking for more detailed information on the process. Until now, I just found out that an eletric arc is made, and to accelerate the process you
can use magnetic camps to shape the spark as a disc (no idea how to do that though). The eletric spark gets the air temperature really high, ionizing
the Nitrogen and Oxygen, and making they react to turn NO. The NO must be cooled down to form NO2 and N2O4, which
will then react with the water to form the HNO3.
So my idea was to put the electrodes inside a flask with two holes. In one of them, a air pumper would throw air inside the flask from time to time
(to let the air converse to nitrogen oxides first and to make pressure on the other hole). In the other hole, a tube would pass through a "condenser"
(to increase the NO2 concentration) and then bubble under a hydrogen peroxide solution in ice bath. Maybe a recycling tube could connect
the unsolved nitrogen oxides and the ionizing flask (but I would have to think how to make it work).
I'm not really informed about the voltage and current information though. I have heard some set ups can form ozone as well. Does the ozone helps on
the process? Where can I find more about those voltage and current set ups?
EDIT:
I'll try to post a sketch on the reactor later, so you have a better idea what I'm talking about.
[Edited on 13-10-2012 by IanCaio]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
N<sub>2</sub>O<sub>5</sub>, produced by direct oxidation was found to be cost-ineffective and electrolytic oxidation is now
popular ─ apparently . . .
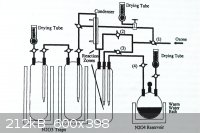
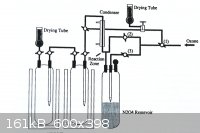
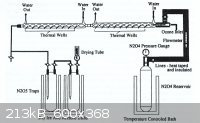
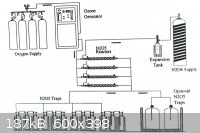
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
If I'm not mistaken, Ammonia (Haber), and Nitric acid are sometimes made together, with a symbiosis which increases the efficiency of each. Platinum
gauze if used to convert air to the ammonia back to NO2. Very little energy is used, as once the Pt gauze is red hot, the rxn is exothermic self
supporting, and the platinum continues to glow. Feedstock is air/water.
Also, I believe this reaction, as of the late 50's and early 60's, has been close to state of art. These plants run 24/7, and are one of the
underlying reasons food can be so cheep in the US. Ammonium Nitrate was probably the easiest to make.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
I'm not sure if I understood, but if I produce ozone as a by product it will then oxidize the NO2 to N2O5. But I'm
assuming it will not interfer on the process since it will sublime, and react with water as well to produce HNO3. Unless maybe it
solidifies in the condenser, but in this case, maybe some water vapour could dissolve it, and it would increase the HNO3 concentration.
I guess I found a guy on youtube, that made a project which was based on this ozone by-production. He was making some N2O5
crystals I guess, and he would then dissolve them into water. But it was a way more complicated reactor..
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
I posted a pic in the Pretty Pictures thread, on page 22
https://www.sciencemadness.org/whisper/viewthread.php?tid=14...
and also, youtube.
This is a test I did at first to see if the concept works at all. I was surprised how quickly the retort gets filled with brown gas. That's why I went
with the big jug (the picture on the Pretty Pictures thread)
http://www.youtube.com/watch?v=eTDLr7L_yEA
Other than that, I am saddened to say that the flyback transformer on the TV is fuming. Too much current passing through. I wish I had kept my neon
sign transformers.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The discharge produced by a Jacob's Ladder set-up will, at its lowest point (spark gap 2-3mm) produce a blueish arc which increases in luminosity, and
changes colour to yellowish, as it travels upwards.
Ozone formation can be expected to be at its max. at the lowest point and conversely, NO<sub>2</sub> at the highest point, where the arc
produces a momentarily snaking motion.
Efficiency is obviously reduced by the poor thermal stability of O<sub>3</sub>; NO<sub>2</sub>, being stable to ~600°C will
be less affected ─ and <i>some</i> oxidation will occur!
Since overall efficiency is of less concern to the amateur than it is to industry, the process has its attractions where near-anhydrous
HNO<sub>3</sub> is difficultly obtained . . .
The use of a capacious envelope to contain the arc will increase efficiency somewhat as cooling rates will be increased!
|
|
|
triplepoint
Hazard to Others
  
Posts: 127
Registered: 11-4-2012
Location: U.S.
Member Is Offline
Mood: in equilibrium
|
|
vmelkon: thanks for the thread. It may have inspired me enough to get me off my a@@ and try it myself. I have a salvaged microwave oven transformer,
that I have been too lazy to rewind. Since this process requires high voltage and low amperage, it seems like the rewinding will be much easier than
some other projects I have considered since I can use thin wire which is easier to work with.
Also, the following is a link to a YouTube video of someone who used this process to make nitric acid. The video and the setup could use some
refining, but they are a successful proof of concept. It can be done successfully with simple equipment.
http://www.y/watch?v=xYrl8mPg8Ag&feature=youtube_gdata_player
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Here's some igniters that don't mind getting a little wet.
http://www.youtube.com/watch?v=NmHW65093_o
I once ran a new little CM-6 spark plug inside a piece of aluminum arrow tubing, it fit snugly inside one end and after a few minutes of running I
took it out and it was very badly eaten up and rusted looking. I was just testing a 10,000 volt Emco unit at a low 6 volts to see the device would run
for long periods without getting hot and didn't expect the new plug to be so damaged. I guess the ozone and oxides of nitrogen would necessitate inert
electrodes for best results.
http://www.youtube.com/watch?v=d33_Fhgy5Dg
I wonder if a bunch of little spark gaps rather than one big spark would make more NO2? As an aside, here's an article that says N2O is the principal
nitrogen oxide generated by a corona discharge.
http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=41...
http://www.youtube.com/watch?v=jMt4RpFd-pw
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | As an aside, here's an article that says N2O is the principal nitrogen oxide generated by a corona discharge. |
The 'N<sub>2</sub>O' is a typo . . .
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Fennel Ass Ih Tone,
Sorry man, I was writing when you posted, didnt see it.
This sounds nice, to be able to do both ammonia and nitric acid, incresing efficiency. But I guess the platinum would be a problem too me. Too
expensive material.
vmelkon,
Nice picture and video. They give a good hint of the overall idea of the project. The flyback is fuming because its being fed with the wrong current?
hissingnoise,
About the anhydrous HNO3, you mean on the N2O5 production right? In the Jacobs Ladder there would be some ozone formation on the
bottom (higher amperage?) resulting in some N2O5 as by-product? In case of a single high voltage spark, would the ozone produced
be something to consider? The higher the current the higher the O3 production?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | The higher the current the higher the O3 production? |
No, O<sub>3</sub> formation is most strongly favoured by 'cold discharge'; NO<sub>2</sub> by arcing in the range; 2000 ─
3000°C
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | | Quote: | | As an aside, here's an article that says N2O is the principal nitrogen oxide generated by a corona discharge. |
The 'N<sub>2</sub>O' is a typo . . .
|
Well if that's true then they got the written word nitrous oxide wrong as a typo in the corona discharge article too.
I found this article on N2O as well.
"Global estimates of nitrous oxide production by lightning and by point discharges beneath thunderclouds are only of the order of thousands of tons of
nitrous oxide per year; however, estimates of global production of N2O by electrical, high voltage, power line coronas are from 1 to 2 orders of
magnitude larger than by lightning."
http://www.agu.org/pubs/crossref/1984/JD089iD01p01411.shtml
[Edited on 14-10-2012 by Morgan]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The <b>principal</b> nitrogen oxide produced by corona discharge is <b>nitric</b> oxide (NO) . . .
It is oxidised to NO<sub>2</sub> on contact with oxygen!
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by IanCaio  | vmelkon,
Nice picture and video. They give a good hint of the overall idea of the project. The flyback is fuming because its being fed with the wrong current?
|
Probably too much current is going through it. I did separate the electrodes as much as could so that most of the heat energy would be dumped onto the
air. Normally, as a TV operates, the + electrode is just there to supply a strong + charge. The amount of electrons being shot out of the canon (the
electron canon in the CRT) is low and doesn't cause heating problems for the flyback transformer. Damn, this sucks  . I knew it would happen. . I knew it would happen.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I have a microwave transformer somewhere in my stuffs, can I make a suitable power supply with that? If yes does anybody have a schematic?
Thanks!
I never asked for this.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
WOW! I tried to make some arc with the microwave transformer and keep the gas. I make a 2 second arc and 2 minutes after the flask was really brown,
much like when dissolving copper. This make a shitload of nitrogen oxide! Now I need to make a suitable reactor, any ideas?
I never asked for this.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Congratulations!
All you really need is to have a large vessel and have some water in there. If you can build a complex system like having a pump to bubble the gas
into the water, it would go faster.
[Edited on 18-10-2012 by vmelkon]
|
|
|
Migratory
Harmless

Posts: 15
Registered: 30-4-2012
Location: Chemophobialand, California
Member Is Offline
Mood: No Mood
|
|
What is this process called? Is it the Birkeland-Eyde process?
|
|
|
| Pages:
1
2 |