| Pages:
1
..
4
5
6 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have finished reducing 1 gallon (3875mL) of "triple distilled white vinegar," which is 5% acetic acid, to a much more concentrated form: 87%. It is
not yet glacial (99+%) acetic acid but it's definitely getting there.
I made this in 3 batches. The 1st was sort of a hodge-podge of CaAc + NaAc. The NaAc was taken back from a diluted HAc. So this batch (30mL) wasn't
too efficient.
The 2nd batch was made from NaAc taken to dryness at about 140C. This yielded about 50 mL.
The 3rd batch was the same as the 2nd only I fused (melted) the NaAc first, then let it solidify, then reground it to a powder. This last step, I
think, was a waste of time as it didn't change anything. This also yielded about 50 mL. I combined all distillates for a total 130 mL for a yield of
(0.87)(130)/[(3875)(0.05)]x100% = 58%. It would have been better if I hadn't messed around with the 1st batch.
Each batch was done with about 80g of anhydrous NaAc and 60mL of concentrated sulfuric acid (Rooto) in a 500 mL RBF assembled to a simple distillation
setup.
In all cases I placed the RBF with powdered NaAc in an ice-bath while adding pre-chilled (w/ice-water) acid. There is a lot of heat generated during
this step. Also there is a lot of smoke generated. I don't know what this smoke is. Once this subsides I heated the mix with a bunsen burner as
required to get a steady generation of HAc collected as a distillate.
I also noted what others have: generation of a lot of carbon black. Also there are smoky odors like that of a wood fire. Perhaps this is some of
the "empyreum" spoken of by the early chemists?
This preparation was a lot of work. It has to be a labor of love as you can buy glacial acetic for reasonable prices. At least I can say that this
method beats my previous preparation with calcium acetate and HCl which yielded 65% acid.
[Edited on 6-10-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Not satisfied with 87% acetic acid I continued my quest for homemade glacial acetic acid. I tried to freeze it to glacial plus eutectic solution by
taking it down to -26C. It froze alright but the eutectic solution was occluded in the acid and therefore was not separable.
So I went back to trying fractional distillation. I packed my little 8" (20cm) column with broken glass pieces from a smashed food jar. I collected
three clear distillate cuts: the 1st was 20mL at 101-104C, the 2nd was 45mL at 104-110C, and the 3rd was 50mL at 110-116C. I left a few mL of brown
solution in the pot. So, it is tough to get glacial acid. If I would have had a longer column and glass raschig rings (or other optimized packing)
I might have done better.
It amazes me that by concentrating something I put on my salad I can make this really wicked (and useful) reagent. 
[Edited on 7-10-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Freezing must be done at a controlled rate. For some materials a very slow decrease in the temperature will result in the excess componant freezing
out in a fairly compact layer, but not always.
The alternative is to freeze to a slush, and vacuum filter or centrifuge to separate the frozen from the eutectic.
Even after freezing distillation is a good idea, as it cleans the acetic acid up. Even if you distilled it at the start, from adding acid to acetate,
if there was more than a few percent of water then other organics will come over in the distillate.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@not_important: Thanks for the information on freezing. I was wondering if there was something I was missing.
Yes, the distillation was good just to clean up the acetic acid. There was a fair amount of carbonaceous junk left in the pot. No wonder the
commercial white vinegar is "triple distilled."
I made another attempt to enrich the 2nd cut by fractional distillation but was only mildly successful. My final product is 65 mL at 92.5% acetic
acid, which I plan to use for an esterification. I've run out of ideas on how to get it any richer.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Could try dehydration with neutral salts, silica gel, molecular sieves, and so on. Also, I believe that fused (anhydrous) sodium acetate will grab
onto the water in the acid. But 90+ percent is fine for most esterfications.
When you distill a mixed solution of the lower alphatic carboxylic acids, a mixture comes over. It will be enriched in some of the acids, but by no
means pure ly one acid. But for food use, a small amount of those acids besides acetic, and the other organics, just make a slightly richer taste.
It's only we fussy chemists how are bother by it.
If you make sodium or calcium acetate as a step in the process, extracting the dry salt with on orgaic solvent might remove some of the larger
organics. As an example, boiling down sodium acetate from vinegar and sodium hydroxide/carbonate/bicarbonate will usually give an off-white to tan
product. Stirring thiis with "100%" isopropyl alcohol removes much of the coloured material.
|
|
|
16MillionEyes
Hazard to Others
  
Posts: 153
Registered: 11-3-2007
Location: 16 Million Eyes, US
Member Is Offline
Mood: No Mood
|
|
I see most of the effort has been done through distillation. Has anyone tried using god ol' home-plausible process of freezing the vinegar and then
collect some of this liquid as it thaws?
I've done it and a weekend with an irritated respiratory tract proved me that this easy process gives off concentrated acetic acid. The only problem
is, how can I accurately measure the concentration given based on the rate at which acetic acid thaws compared to that of water?
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Summary of above thread
(1)The mysterious azeotrope of the acetic acid/water system is as elusive as the blue-arsed fly; it does not exist. The system is zeotropic.
(2)You can increase the % acetic acid in a solution by distilling and get 55% or better with effort (several distillations)
(3) "Pure" or glacial: good luck. Try freezing a well concentrated solution?
For reference, graphs of vapor (partial) pressure vs. temp are attached to help (all data plotted from CRC 1995-1996 ed.)
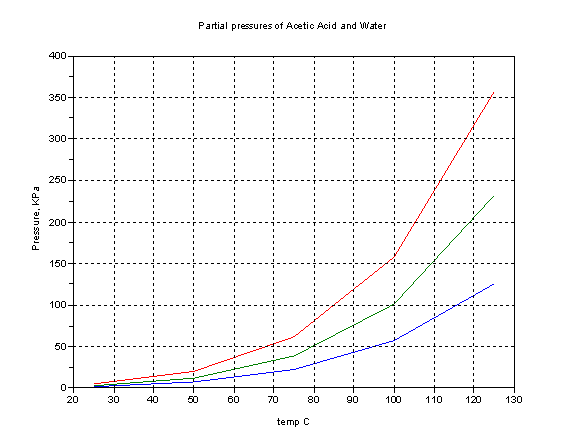
Lower trace, acetic acid, middle water, top sum of the two. 101 Kpa ~ 1atm. =760 mm Hg. The top trace is not highly meaningful since the presence of
the one solvent in the other reduces the vapor pressure of that component.
The fact that the system is zeotropic tells us that the slope of the boiling temperature of a mixture plotted against composition (0-100% acid) has
no maximum nor minimum but must have a positive slope throughout. Raoult’s Law never holds except for low dilution factors but assuming a straight
line tells you very roughly where a given composition boils.
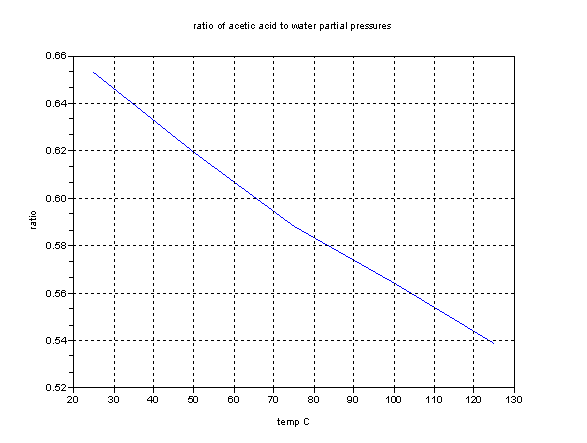
This graph shows the ratios of the partial pressures. Remember that these graphs refer to the vapor in contact with the pure liquid only. But they may
help.
Regards,
DerAlte
[Edited on 28-8-2007 by DerAlte]
[Edited on 29-8-2007 by DerAlte]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This
http://en.wikipedia.org/wiki/Blue_bottle_fly
is a whole lot more common than any acetic acid/ water azeotrope.
I think you missed out the use of involatile salts- like calcium or sodium acetate and sulphuric acid as a method too. It's a whole lot easier to
remove water from sodium acetate than from acetic acid.
Otherwise a useful summary of a remarkably long thread.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@ unionised - LOL! Yup, missed that bit. Better not make an already long thread longer! Regards, Der Alte
|
|
|
merlic79
Harmless

Posts: 10
Registered: 16-1-2008
Member Is Offline
Mood: No Mood
|
|
Why don't you guys just use phosphoric acid? It is readily available in 85% concentrations. As well it is food grade. I mixed it with some dried NaAc
and then did a simple distillation....it VERY pungent. I plan to take this acid and distill with H2SO4 to get pure GAA.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Vacuum crystallization:
US3677023
Note the water remaining when vacuum is not used.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Gee,simply vacuum stripping the product really.I wouldn't have thought such common technique patentable.I suppose the confined temperature range got
it over the line.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
I recently tried the procedure in this patent, starting with about 90% acetic acid and had no luck with it.
I'd be very excited to hear that someone else succeeded!
Better to remain silent and appear a fool than to open your mouth and remove all doubt.
|
|
|
BenZeen
Harmless

Posts: 37
Registered: 15-1-2010
Member Is Offline
Mood: No Mood
|
|
freeze crystallisation of acetic acid
Ok, i have used the FSE and couldn't find much so here goes...
The freezing point of vinegar is -2°c at a conc of ~5% acetic acid, and I have seen a photo thread on another website of a person using freeze
crystallisation to concentrate acetic acid from wine vinegar (www.alchemywebsite.com) and I wonder if it would it be possible to use this technique to concentrate acetic acid from white vinegar (about 5%
conc), or if anyone has done it already? Thanks
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=2194&a...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Nicodem
|
Threads Merged
18-3-2010 at 08:57 |
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
This attachment is from 1918 and the serial book Vinegar Bulletin, by Paul Hassack. It's the interesting part of THE CONCENTRATION OF SPIRIT VINEGAR,
and OTHER CONCENTRATION METHODS OF FERMENTED VINEGAR pp. 210-211.
Or
http://books.google.com/books?id=axQwAAAAYAAJ&pg=PA210
Attachment: Vinegar_Bulletin.pdf (110kB)
This file has been downloaded 677 times
[Edited on 27-10-2013 by S.C. Wack]
|
|
|
| Pages:
1
..
4
5
6 |