IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Question on aromaticity in ions
Hey guys,
I've been away for a while, and for now I'm focusing on the college tests.
I'm actually enjoying organic chemistry, but it's lots of information and I'm running against time, partially my fault  . I'm researching to understand some stuff but this was something I couldn't find. . I'm researching to understand some stuff but this was something I couldn't find.
My doubt is something simple I guess, about the aromatic compounds. We learned the Huckel rule (4n + 2), and that there should be space for the
electrons to move (i.e. sp3 and sp1 carbons are "blockers"). But what about extra electrons in the cyclic structure?
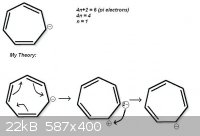
One example on the attachment (If someone knows this ion name please tell me, I've no clue about organic ions nomenclature).
I think its an aromatic compound, but I'm not sure if those electrons can move the way I described in the sketch.
Thanks guys
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
the carbocation would be tropylium. about its aromaticity: the frost circle
[Edited on 15-11-2012 by tetrahedron]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by IanCaio  | Hey guys,
I've been away for a while, and for now I'm focusing on the college tests.
I'm actually enjoying organic chemistry, but it's lots of information and I'm running against time, partially my fault  . I'm researching to understand some stuff but this was something I couldn't find. . I'm researching to understand some stuff but this was something I couldn't find.
My doubt is something simple I guess, about the aromatic compounds. We learned the Huckel rule (4n + 2), and that there should be space for the
electrons to move (i.e. sp3 and sp1 carbons are "blockers"). But what about extra electrons in the cyclic structure?
One example on the attachment (If someone knows this ion name please tell me, I've no clue about organic ions nomenclature).
I think its an aromatic compound, but I'm not sure if those electrons can move the way I described in the sketch.
Thanks guys
|
That structure would not be aromatic, as it would have 8 pi electrons if the negatively charged carbon was sp2 hybridized. If planar, it would be
antiaromatic. I'd call this structure a cycloheptatrienyl anion.
Meanwhile, the cationic form is aromatic. The carbocation assumes sp2 hybridization, the ring has 6 pi electrons, and it has 7 resononance structures.
http://sst-web.tees.ac.uk/external/U0011579/Aromatic/Cyclohe...
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thank you guys!
Edit:
Just traveled here.. So when the pi bond came to the negative carbon, both the free electrons would be pi electrons?
That means it would be aromatic if I had a 9 carbons cyclic anion
with 4 pi bonds, right?
Thanks again 
[Edited on 16-11-2012 by IanCaio]
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
I passed in Organic Chemistry I this semester!
Did better then I expected, thanks for all your help. He didn't ask about aromacity in the test (which I spent a few time studying) but I appreciated
really learning this stuff, and you helped me out with this doubt about the ions, so thanks again for the help!
|
|
|