| Pages:
1
2 |
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
Of course you could use your clothing as well. Belt buckles might dissolve in HCl over time...
Tossing in a phone battery might do enough electrolysis before dying that chlorine could be detected..
[Edited on 2-3-2013 by kavu]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Here's a small modification of the wetting procedure... I've took the photo on my table few minutes ago.

NaOH should climb higher. Now, again, I don't know if 1 M is enough.
Also, drops of the solutions sliding down the glass, bases should behave differently as they adhere to the glass more than acids.
The blood thing came to mind, but it's kind of invasive... And I'd probably start feeling woozy because I can't stand to see my body leaking
from its primary coolant circuit. °_°
It would be interesting to test whether humans could distinguish the sound given by glass rod banging on the beakers with fluids which densities vary
so little. Probably not, and the experimental error would suffer a lot because of minute differences in beakers so any slight effect due to different
fluid densities would be hidden. But it's a nice thought. 
Regarding the light diffraction, yes - we're left with Na<sup>+</sup> and Cl<sup>-</sup> and H<sub>2</sub>O, but
that's after proper mixing. In the first few moments the blob is solution A in solution B, surrounded by a diffusing area of products. Someone should
test whether 1M solutions really do show visible diffraction and floating/sinking. I think it should be visible under optimal lighting conditions.
[Edited on 2-3-2013 by Endimion17]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Tear out a strip of your trousers and but each end in one of the beakers. The HCl and the NaOH would hopefully go up because of capillarity, and they
would react forming NaCl nearer the NaOH beaker (well, I don't know how to describe it, but you can tell the difference).
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Would it work?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
NaOH solution freeze below 0c between 1 and 18% (by weight) HCl sol, will freeze way below that (25% by weight freezes around -80c)
so if you can drop the temperature in the room the first solution that freezes is the NaOH one
no need to cut my self or pee in anything! lol
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
The HCl solution is more volatile than NaOH. Evaporation speed!
Experiment 1:
Combine 10 drops of solution x to form a circle.
Combine 10 drops of solution y to form a second circle.
Use your glass pipe to produce an air current. The circle that evaporates fastest is HCl. No need to wait for total evaporation (which is actually
testing for a solid residue, not volatility).
---------------------------------------------------------------------------
Experiment 2:
Combine several drops from each solution together on the table. Using this test salt solution, drop it into each solution. You may be able to
determine relatively which solution has the greater viscosity (which will be the NaOH) by the way the drops sink (go deeper into the HCl).
Alternate test for Viscosity (more sensitive and easier to measure):
Place a drop from each solution next to each other. Blow on them. The solution that consistently produces the longest track is HCl. Perform at least
20 runs and alternate the right and left solutions on different parts of the table.
----------------------------------------------------------------------
Experiment 3:
Fill equal amounts of both solutions in both ends of the semi-circular glass tube. Balance on the pointy edge of the table to determine which is
relatively heavier. The one with the greater weight is NaOH (higher Specific Gravity).
------------------------------------------------------------------------
Experiment 4:
Sometimes to get a handle on a problem, one has to use his hand. This experiment measures visual distortion by looking at your hand through both
solutions (that is, you adjust your position to line up the solutions visually in a straight line). Repeat with each solution being 1st and then 2nd.
The manner that produces the greatest distortion occurs when the 1st solution is NaOH.
---------------------------------------------------------------------------
Now, if one can use legally use bodily fluids, I will leave this experiment to my illustrious colleagues. Place samples of urine on the table and wait
to some ammonia has formed. Insert the rode into the ammonia solution and wave it over the fluids. The HCl will immediately form smoke clouds of
NH4Cl. (note, this was already cited but most likely not legal nor, one could argue, is using CO2, a bodily exhaust and a reagent).
-------------------------------------------------------------------------------
And yes, you cannot make Cl2 unless you cheat and use some wiring from a lamp, two pennies (anode and cathode) and connect the two bowls with the
tubing (a salt bridge), etc. Now, I will let my esteem colleagues debate over whether blowing air into HCl in the presence of diffused sunlight, a
couple of pennies, makes any HOCl, and then with HCl, equals Chlorine, which would change the solution's color ever so slightly. Or, am I just blowing
hot air at 400 C?
[Edited on 4-3-2013 by AJKOER]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Common AJKOER, I know you would like to use hypothetical way using bleach and vinegar! Bfesser allowed you to use your usual loved reagents.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/9/13 by bfesser]
I never asked for this.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by neptunium  | NaOH solution freeze below 0c between 1 and 18% (by weight) HCl sol, will freeze way below that (25% by weight freezes around -80c)
so if you can drop the temperature in the room the first solution that freezes is the NaOH one |
They're both 1 M, so they'd both contain 2 M ions, and should suffer the same freezing point depression, shouldn't they?
1M NaOH will contain 40 g solute per L, so is 4% by mass; 1 M HCl will be 3.6% by mass.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
No, the freezing point of a solution has its own value according to concentration AND nature of what is disolved in it.
even at same concentration the freezing and boiling point are different for each chemical. (in solution)
HCl freezing point
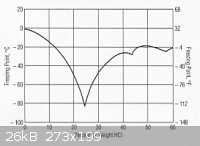
NaOH freezing point
![Na-Freezing-points_sodium_hydroxide[1].jpg - 124kB](https://www.sciencemadness.org/whisper/files.php?pid=276801&aid=22591)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
In dilute solutions, the two don't seem to be very different. They both look like about -4oC for 1 M.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/9/13 by bfesser]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes its true, but 1M of NaOH is 40g/1000ml or 4% by weight
for 4% in each case i read -8C for HCl and about -4C for NaOH that should be enough to make a difference?
i agree less would be difficult though
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
1M HCl is 3.7%, but I can't read that tiny graph well enough to get a more precise freezing point.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/9/13 by bfesser]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
granted its a bit difficult but you can tell the tiny difference between the very visible graph for NaOH and ruffly that of HCl..the function aim at a
different point so mathematicaly you can tell its a different function that drives the curves so when temperature goes down even if 2 or 3 degrees
only seperate the two solution from the freezing point it might take 10 to 20 minutes to happen which should be enough to identify which is which. if
you keep an eye on it
and if you were busy getting drunk watching family guy, and forgot about the experiement and find both frozen solid after 5 and a half hours then you
can always get a second chance watching them thaw!
and sober up!
[Edited on 4-3-2013 by neptunium]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
| Quote: | | Tear out a strip of your trousers and but each end in one of the beakers. The HCl and the NaOH would hopefully go up because of capillarity, and they
would react forming NaCl nearer the NaOH beaker (well, I don't know how to describe it, but you can tell the difference). |
... Would it work?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Wait a few days. The one that has evaporated more should be HCl.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
"A Hypothetical Chemist"
Would that be an Hypothecary?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Yes, yes it would.
As usual, everyone overlooks the simplest solution:
<em>Ask the technician!</em>
[Edited on 7/9/13 by bfesser]
|
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
Couldn't something be done with HCl(gas) solubility, such as when dissolving HCl in water, significant back flow occurs.
Possible Experiment:
Stick glass tube in to solution, one end covered, the air space in the tube should contain some HCl fumes, then blow away extra fumes from the rest of
the solution, then after a time the liquid level will rise in the tube as it tries to absorb the extra HCl in the airspace of the glass tube?
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Not sure if this counts as "extra chemicals", but you could regurgitate the red cabbage soup you ate for lunch (filter if necessary  ) and add some to each of the beakers. IIRC, the HCl will go red, and the NaOH
yellow/green? ) and add some to each of the beakers. IIRC, the HCl will go red, and the NaOH
yellow/green?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Wilhelm Scream
Harmless

Posts: 2
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
Not sure if this method is already offered, but whatever.
Hold one end of the glass tube in the solution, close the other hole with your thumb. If it's hydrochloric acid it should fill up with HCl fumes. Now
quickly take out the glass rod, and close the other hole with your finger. Then put one opening in the other solution. If it's the basic solution the
water should be sucked into tube, because dissolving/reacting the HCl fumes will lower the pressure in the tube.
If nothing happens, do it over with the order of the beakers reversed, just to be sure.
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
Lick both solutions, the sour one is HCl.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Read the OP post - olfactory senses have been disabled. I think that includes taste.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/9/13 by bfesser]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by Eddygp  | | Quote: | | Tear out a strip of your trousers and but each end in one of the beakers. The HCl and the NaOH would hopefully go up because of capillarity, and they
would react forming NaCl nearer the NaOH beaker (well, I don't know how to describe it, but you can tell the difference). |
... Would it work? |
Would NaCl crystallize more easily than NaOH and therefore leave a mark on the place where the reaction happened?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
| Pages:
1
2 |