| Pages:
1
2
3 |
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I would think that it would be rather explosive, considering what the thermodynamics of decomposition would probably be.
I weep at the sight of flaming acetic anhydride.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I don't know exactly what the composition of the explosive material is, given that Muspratt doesn't know and I don't have a good inorganic reference
at my current location. I do know that texts on the carbothermic production of potassium warn that the black material you get along with potassium
metal when you strongly heat carbon and potassium carbonate together is explosive and should be removed from the metal as soon as possible.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
I wonder why anybody is seeking for difficult methods when pentanitroaniline (probably) can be made starting with aniline (I am almost sure).
edit: added <i>probably</i> and <i>almost</i> 
[Edited on 23-7-2003 by KABOOOM(pyrojustforfun)]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Could you provide details on preparing pentanitroaniline from aniline?
I weep at the sight of flaming acetic anhydride.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
from my chemical dictionary:
trinitroaniline (picramide)
C6H2NH2(NO2)3.
Properties: Orange-red crystals; m.p. 188°C; b.p. explodes; sp.gr.
1.762.
Derivation: Nitrating aniline in glacial acetic acid solution or by the use of mixed nitric-sulfuric acid in limited amounts.
Hazard:
Dangerous; explodes by heat or shock.
Use: Explosive compositions.
Shipping regulations: (Rail) Explosive A label. Not acceptable passenger.
(Air) Not acceptable dry, or wet whith less than 10% water; Flammable Solid label when wet with not less than 10% water.
tetranitroaniline
(TNA) C6H(NO2)4NH2. A nitration product of aniline which melts at 170°C and explodes at
237°C.
Hazard: Toxic. Dangerous fire and explosion risk.
Use: Manufacture of detonators and primers.
Shipping regulations: Detonating
primers, (Rail) Explosive A label. Not acceptable passenger. (Air) Not acceptable.
so the usual mixed acid nitrates it to tetranitroaniline.
the only -H which is not nitrated should be at 5th C. knowing that the 3d C is nitrated, it's perhaps possible that at hardrer conditions the
symmetric position (5th C) could be nitrated.(I'm not completely sure)
I've thought of another method starting from chloranil (see the
attachment)
sorry for late replying ,unfortunately I won't have many posts until summer. (those who had ICQ chat with me know why).
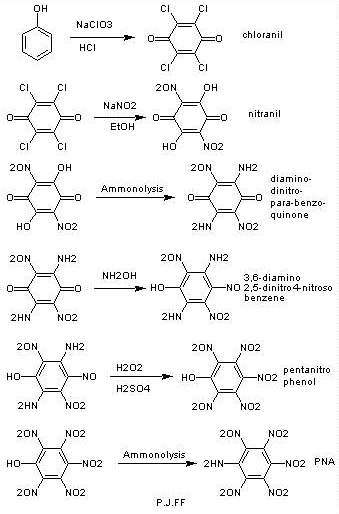
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Re:Hexanitrobenzene
Hexanitrobenzene: Heat of formation: (in cal/g) 12, Density: 0.0717 in
lbm/in^3 -in NASA thermochemistry you would need to convert from
cal/g to cal/mol by multiplying these values by the molecular weight of
the compound. this info is taken from the good ol' Propulsion data
base:............................solo
http://roger.ecn.purdue.edu/~propulsi/propulsion/comb/propel...
which also has more valuble information like this on other compounds.
NO2
|
|
/ \
/ \
/ \
NO2¯¯¯¯| |¯¯¯¯NO2
| |
NO2¯¯¯¯ \ / ¯¯¯¯NO2
\ /
\ /
|
|
NO2
Hexanitrobenzene
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
forget about chloranil method even if it could work untill the pentanitrophenol part. the next step probably won't work because amminolysis usually
needs high temperature and PNP may decompose at that temp.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The procedure to form HNB that I mentionned is from a book dedicated to pernitrocoumpounds
-TNMethane
-HNEthane
-TeNEthene
-HNB
Are discussed extensively but some unknown compounds were discussed too (ONC has been synthetised meanwhile)
-octanitropropane
-Dinitroacetylen
-octanitronaphtalene
-ONC
....
They mention that dinitrocetylen has been tried without succes for decades!
That HNB is very sensitive towards moisture in wich it turns fast into trinitrophloroglucidol
C6(NO2)6 + 3H2O --> C6(OH)3(NO2)3 + 3HNO2
This explains why the final step of the reaction of tetranitrotoluidine must be carried out in anhydrous HNO3/H2SO4/H2O2!
So one of the way to produce HNB is via Toluen!
C6H5-CH3 + HNO3 --> CH3-C6H4(NO2)
para nitrotoluene -Fe/HCl-> Paratoluidine
CH3-C6H4-NH2 -HNO3/H2SO4-> TetraNitroParaToluidine
CH3-C6(NO2)4-NH2 -HNO3/H2SO4/H2O2-> HNB + CO2
The final step is peroxydation of the amino group to a nitro one, oxydation of the CH3 into CO2H, decarboxyaltion and nitration of the resulting H!
I don't know if the ammonolyse of the nitrophenols is easy to perform at moderate T (under the T of detonation of such powerful and sensitive
HE)!
Anyway, I have to correct some errors that I have seen along all this chat!
-NH2 is a ORTHO and PARA director with a favor to para depending on the substituant!
-NH3(+) is a meta director (this explains why some TeNA is formed in the TNA process!
-Aminobenzene will produce very little TNA,TeNA or PNA by direct nitration without any protection since aniline readily oxydises to para quinone.This
is circumvented in the case of paratoluidine because of the CH3!
-In the same order of idea paradiaminobenzene will perform very bad (as pararesorcinol) in nitrations; usually you get complete decomposition
(oxydation in quinone and then water and CO2 + nitrous fumes)!
-It is possible to make paradiaminotetranitrobenzene via paradichlorotetranitrobenzene! The fact two groups are para to each other is absolutely not a
problem!Thus nitrating pDCB is as simple as nitrating MonoCB and a little less than metaDCB.In the case of paratoluidine the NH2 is stronger activator
than CH3 and will rule the two first nitro in ortho to the NH2; then the CH3 activates the position in ortho to itself; the two effects are not
concertative but doesn't oppose really on each other!
To understand this:
speed of nitration of benzene is 1
speed of nitration of toluene is 10
speed of nitration of metaxylen is 30
speed of nitration of orthoxylen is 15
speed of nitration of paraxylen is 20
speed of nitration of mesitylene is 100
Thus no mather the position of the activating group towards each other, they activate more when there are more and even more if they are concertative
(in meta of each other)!
Chlorobenzen nitrates almost as fast as benzen so DCB will do aswel and as in the case of benzen to go high in NO2, you need SO3 oleum and anhydrous
HNO3!
The synthesis of Cl-C#C-H from CCl3-CH3 and Al seems doubtfull since Al in polyhalide is a wish for a detonation (heat sensitive)...so dehalogenation
in explosive Cl-C#C-H seems very dangerous...even more if applied to the hypothetical (di)nitroacetylen synthesis!
Anyway one might attempt to "condensate" chloropicrin with Al...?
2O2N-CCl3 + 2Al --> O2N-C#C-NO2 + 2AlCl3
Nitroacetylen and dinitroacetylen are more than certainly subject of strong instability due to nitronate resonance and nitrito resonance!This effect
explains the poor stability of tetranitroethylene!
O2N-C#C-H <-->O=N-O-C#C-H <--> HO-C#C-N=O <-->
O=C=C=N-OH
I personnally would be very cautious with any attempts to produce dinitroacetylen since most precusor are already explosives!
I could think to
O2N-C#C-NO2 from I-C#C-I and AgNO2, from C2H2 and I-NO2 to form I-C#C-NO2 and then subsequent action of AgNO2
Cl2C(NO2)-C(NO2)Cl2 refluxed with Zn in an inert solvant!
Note that pernitrocompounds are strong nitrating agents so solvant has to be very unreactive!They also display a positive OB and free NOx so they are
panclastite like HE mixes with combustible solvants!!!!Heat, shock have to be avoided at any costs!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
If hexanitrobenzene is so sensitive to moisture, nitrating in dichloromethane might be an interesting option. An abstract and a patient mention DCM as
a relatively safe and inert nitrating solvent which also seems to promote yields in aromatic nitrations.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
R-Salt
Harmless

Posts: 3
Registered: 14-6-2003
Location: Sierra Nevada, Spain
Member Is Offline
Mood: No Mood
|
|
Hexanitrobenzene
Simplest would seem to be :- follow standard method to 1,3,5-triamino 2,4,6-trinitrobenzene then use 98%h2o2 in oleum to oxidise -nh2 to -no2. An
interesting alternative would be starting from hexanitrosobenzene (available ) which is a tautomer of benzene trifuroxide. Furoxides are known to be
oxidisable to nitro with peracids.
Does anyone happen to know if the pyridine equivalent of HNB has been made?. pentanitropyridine
|
|
|
R-Salt
Harmless

Posts: 3
Registered: 14-6-2003
Location: Sierra Nevada, Spain
Member Is Offline
Mood: No Mood
|
|
Hexanitrobenzene
It occurs to me that it might be possible from triaminotrinitrobenzene as follows:-
RNO2 to diazotise the three NH2 then replace N2+ with NO2. The "normal" NaNO2 / HNO2 aqueous would obviously be no good as HNB is unstable
to water, but alkyl nitrite then a non aqueous NO2 replacement might work.?
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
as -NO2 groups increase in the ring, diazotation of the amino group becomes harder for example diazotation of picramide needs conc sulfuric acid. if
TATB could be diazotated the othere amino groups will become very hard/impossible to diazotate. there are many disadvantages in oxidization of TATB,
firstly it's insoluble in most (any?) solvents. secondly @ least three times more H2O2 & H2SO4 is needed than the usual method (using PNA as
starting material) . TATB is rather hard and expensive to make (although the VNS method via NH<sub>2</sub>OH makes it cheap & easy).
I'm very hopeful that VNS amination of TeNA is possible . this will yield 1,3-diamino 2,4,5,6-tetranitrobenzene. DATeB is a very powerful
explosive on its own (definitely more powerful than TATB). its oxidation hopefully results HNB. a alternative method for oxidization step is to add
porassium persulfate in H2SO4 instead of adding conc H2O2 in it.
[Edited on 23-7-2003 by KABOOOM(pyrojustforfun)]
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
US patent 4,262,148 describes the process for synthesizing HNB from pentanitroaniline, here's the abstract, followed by a summary of the process:
Hexanitrobenzene is prepared by oxidizing the amine group of pentanitroaniline with H2O2 in H2SO4 The compound is a high density explosive.
The process is something like dissolve 1 gram of pentanitroaniline in H2SO4 with 20% dissolved SO3 content. After cooling to 5 degrees C, slowly add
5 mL of 98% or higher H2O2 keeping the temperature under 30 degrees C. The solution is protected by a drying tube and kept at 25 degrees C for 24
hours, then at 0 degrees Celsius for one hour, after which the precipitate is removed.
US patent 4,248,798 describes how to prepare pentanitro aniline from TNT here is the abstract, followed by a somewhat lengthier summary of the
process:
Trinitrotoluene is selectively reduced by reaction with H2S in p-dine to produce 4-amino-2,6-dinitrotoluene. This latter compound is then nitrated
with HNO3 in H2SO4 to produce pentanitroaniline.
This process involves dissolving 25 g of TNT in 50 mL of p-dioxane that contains 1 mL of NH4OH as a catalyst. H2S is bubbled through for 30 minutes,
and there should be a precipitate. The soution is filtered and the precipitate which is a mixture 4-amino-2,6-dinitrotoluene and
2,6-dinitro-4-hydroxylaminotoluene is recrystallized from methanol to get the 4-amino-2,6-dinitrotoluene. 1 gram of 4-amino-2,6-dinitrotoluene is
dissolved in 40 mL of 96% H2SO4 and 3 mL of 90% HNO3 are added dropwise, while the reaction temperature rises to 40 degrees Celsius. It is then
heated for one hour at 70 degrees Celsius. The solution is then cooled to room temperature after which the acids are extracted with methylene
chloride and the extract is dried over MgSO4. After the methylene chloride has evaporated a 62.4% yield of pentanitroaniline is obtained.
Read the patents if you want all the gritty details, but that was most of them.
[Edited on 25-12-2003 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
Mendeleev: we knew both patents. fully read the thread?
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Whoops... Terribly sorry about that, sort of missed that first page there  .
Thanks for telling me otherwise I would have gone on in ignorance. .
Thanks for telling me otherwise I would have gone on in ignorance.
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
polyhydroxycyclohexanes as intermediates
Has anyone thought of using hydrogen peroxide in any of this?
resocinol and H2O2?
I think that the H2O2 will split the double bonds in a benzene like structure.
C6H4(OH)2+3H2O2-->C6H4(OH)8
From ther you can nitrate it.
It's not hexanitrobenzene, but its similar . .
Also, p-nitrophenol+gallic acid. Oooh! Add H2O2 and nitrate! Wowwwww!
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Turel
Hazard to Others
  
Posts: 141
Registered: 29-11-2003
Location: The Hardware/Software Interface
Member Is Offline
Mood: Thixotropic
|
|
No
Peroxide will not effect benzenoids that way. Aromaticity complicates matters (or simplifies them, depending on how familiar you are with organic
chemistry).
Resorcinol could be oxidized in small yields with H2O2 to meta-benzoquinone. Resorcinol is rather inert to H2O2 in low concentrations. If benzenoids
were nonaromatic, you would still produce a different compound using this method. (cyclohexan-1,2,3,4,5,6-hexol hexanitrate).
|
|
|
Spawn
Harmless

Posts: 1
Registered: 7-5-2004
Location: everywhere
Member Is Offline
Mood: No Mood
|
|
hexanitro derivates ???
Hexanitrobenzene:
Hexachlorobenzene + ammonia + Soium Acetate
---> Hexaaminobenzene
Hexaaminobenzene + H2SO4 + H2O2 (Caros Acid)
--->Hexanitrobenzene
Hexanitrocyclohexane:
Hexachlorocyclohexane + ammonia + Soium Acetate
---> Hexaaminocyclohexane
Hexaaminocyclohexane + H2SO4 + H2O2 (Caros Acid)
--->Hexanitrocyclohexane
 
|
|
|
rsgpit
Harmless

Posts: 14
Registered: 19-5-2006
Member Is Offline
Mood: No Mood
|
|
Different Nitrobenzene
Would it be possible to have a nitro group of each carbon in a benzene ring. I know regular NB has only one Nitro group and 5 hydrogen. Has it ever
been done? If it has, its surely very powerful, yeilding only CO2 and N2.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
Wouldn't that be hexanitrobenzene? The first search returned in Google, a very useful tool, gave this:
http://en.wikipedia.org/wiki/Hexanitrobenzene
It is a powerful explosive, but is apparently sensitive to light !
 
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Also susceptable to hydrolysis in the presence of moisture... it is not chemically stable, however it is possible to prepare as long as moisture is
avoided and it is not left in the light.
It's explosive properties are amoung the most powerful of any explosive prepared to date. I believe it is more powerful than CL-20 by a decent
margin, though don't have any refs at the moment.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I was always put off by benzene as Gerald Hurst was one of the people back in the early 1990's who claimed it was possibly one of the things that
contributed to his health issues. I remember having received some from a chem supplier a long time back and it's MSDS was one of the only ones that
claimed that it (in and of itself) was a very serious carsinogen; that it was a prooven cause. Jerry claimed that he used it to clean up with and his
liver funtion tests in his mid 50's was very seriously poor. That being said I believe that Marshall has some information about it's extended
nitration. Obtaining it today may be even more difficult than 10years back. Way back then I was interested and may have some direct info re same -
will post if I can dig it up.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
How would one prepare hexanitrobenzene, presumably starting with nitrobenzene, and especially given its sensitivity to light and hydrolysis moisture?
It would be very difficult to nitrate it beyond 1,3,5--trinitrobenzene, because after that the less favorable electrostatic charge distribution, and
steric strain, makes further electrophilic substitution with further -NO2s unfavorable. To avoid moisture, one would have to use something like N2O5
or NO2ClO4, rather than HNO3 with H2SO4.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Toluene -> TNT, selective reduction of the 4-nitro with H2S, then oxidation/nitration to tetranitroaniline (high temps to oxidize the methyl to
COOH and then de-carboxylate it), and finally oxidation with H2SO4/H2O2 (100% or fuming and 98% concentrations respectively).
I think the final oxidation step could be improved and made more simple, for example a H2SO4/Na2WO4/H2O2 mixture, which is more active and efficient
at oxidizing NH2 groups, especially deactivated ones. It would require much less oxidizing mixture, and give higher yields as well. Maybe toss some
SO3 in the form of fuming sulfuric acid to improve the yield by removing all traces of water.
Also, thinking to picric -> picramic reductions, perhaps ascorbic acid (vitamin C) could be used to selectively reduce the TNT without the horrid
smell and chance of death.
Then, after isolation, it's time to brag big time on the internet 
|
|
|
Madandcrazy
Hazard to Others
  
Posts: 117
Registered: 11-5-2005
Member Is Offline
Mood: annoyed
|
|
Nice idea hexanitrobenzene by TNT, but how prepared PNT (pentanitrotoluene) and how oxidized/substituted to HNB ?
Are oxidzing around a good method, i think it is some wasteful and poisonous.
TNT -->
1-methyl-2-amino-4,6-dinitrobenzene -->
1-methyl-2-amino-3,4,6-trinitrobenzene -->
1-methyl-2-3,4,6-tetranitrobenzene -->
1-methyl-5-amino-2-3,4,6-tetranitrobenzene -->
PNT
But how oxidized PNT to HNB (PNA), with a mix of a hyposulfide/chloroamin or NH4OH/hyposulfide ?
Maybe TNT is strong treated in a vessel in low amounts with HNO3/H2SO4 to PNT or to trichlorotrinitrotoluene  . .
NaN3 is evantually useful as a buffer subtsance for substitution/nitration.
Is trinitrobenzene not even treated by this method to HNB ?
More capabilities maybe:
chloropicrin is treated to chloropentanitrobenzene and this is continued with ammonium acetate to pentanitroaniline.
hexachlorobenzene is treated with the same.
I think some interest applies to for syntheses of pentanitrophenol or some salts like ammoniumpentacrate  , but some of this high nitrated salts still stable ? , but some of this high nitrated salts still stable ?
Excuse me, when some nomenclatura not correcly in this long post.
[Edited on 2-6-2006 by Madandcrazy]
|
|
|
| Pages:
1
2
3 |