Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
isophorone diamine
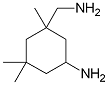
I have a can of epoxy hardener that contains isophorone diamine together with benzyl alcohol.
Over time, this hardener deteriorates, releasing ammonia, NH3. The epoxy formed will then be of a lesser quality than when the hardener is fresh.
I wonder how the isophorone diamine could be separated from the deteriorated hardener. Both isophorone diamine and benzyl alcohol dissolve in water;
isophorone diamine is miscible (data sheet), benzyl alcohol dissolves at 4.3 g/100 ml (wiki). Could mixture with water lead to preferential solution of the amine, leaving the
alcohol in a separate layer?
Isophorone diamine boils at 247 centigrade, but it decomposes at 260. Benzyl alcohol boils at 206 centigrade, so I might try careful destillation. I
fear that the diamine would undergo some self polymerisation, though, a tendency it already seems to show at RT over time.
Any other thoughts for separation? Addition of base/acid, maybe?
Could isophorone diamine be used as a complexing agent for metal ions, similar to ethylene diamine?
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I can imagine that adding an acid may protonate the amine and that you could be able to crystallize the salt of this, such as the .2HCl or .H2SO4 salt
of it. Such a salt then could be interesting to use in experiments. Simply adding a base like NaOH would release the free amine and then you can do
experiments with metal ions (e.g. copper most likely gives a deep blue complex with this). For such experiments I would try to crystallize the .H2SO4
salt, because sulfate ion has much less tendency to coordinate to all kinds of metal ions than chloride ion.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
I did two simple tests with the expired hardener. This is a somewhat viscous light yellow transparent mixture. First, I put some of it in a fridge, as
the melting points of isophorone diamine and benzyl alcohol are 10 °C and -15 °C resp., hoping that the diamine would solidify. After one day this
had not happened. Instead all of the yelow matter had become a transparent, somewhat sticky solid.
The second test was to mix some of the hardener with water, to see whether it would indeed dissolve. This did not happen. Some 5ml of water can be
mixed with 20 ml of the hardener, but when more water is added, a suspension is obtained after shaking, that separates again on longer standing.
After standing for one day, I separated the mixture by filtration with paper, discarding the majority of the lower yellow viscous layer, and keeping
the upper more or less clear liquid. I will check whether addition of some H2SO4 will yield a precipitate. It could well be that almost all of the
hardener has undergone self-polymerisation, and does not contain any appreciable amount of isophorone diamine anymore.
Glassware needs to be cleaned with acetone.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
To separate it from any non-amine ingredients you could precipitate the hydrogen oxalate or oxalate salt by adding an acetone solution of oxalic acid
(dihydrate) to the diamine in acetone. You may need to heat it slightly to form the oxalate salt, which will most-likely crystallize from the cooling
solution.
[Edited on 17-9-2013 by sonogashira]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Separation with H2SO4 - failed
I tried the method woelen suggested as follows.
1. Mix some of the hardener in water (volume ratio hardener:water ~ 1:8). Shake or stir vigorously, with the idea that the diamine can fully dissolve
in the water.
2. Let stand, so that the benzyl alcohol will largely separate.
3. Decant, keeping the colourless watery part.
4. Add 2 ml of 96% H2SO4 to 40 ml of this colourless solution. The idea being that the sulfate of isophorone diamine will crystallise from the
solution.
In step 4 a characteristic smell was noticed, similar to that of the hardener itself, suggesting the presence of at least one of its components. The
solution heated up when H2SO4 was added, and became cloudy. On standing, no precipitate was formed.
Standing in a refridgerator overnight (~ -7C), froze the entire solution.
Question 1: is there anything I did wrong in the method?
Question 2: I noticed that the yellow layer, supposedly containing most of the benzyl alcohol, dissolved very well in blue spirits (which turned a bit
greenish). Is it worth trying to add H2SO4 to an ethanolic solution of the hardener, to separate out the sulfate of isophorone diamine? Is it okay to
use ketonated alcohol as the solvent, instead of pure alcohol (compatibility wigh reaction)?
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
The protonated form (R-NH3<sup>+</sup> is likely to be more soluble in
water. is likely to be more soluble in
water.
I think it would therefore be better to add the sulfuric acid already in step 1, then dissolve as much as you can. Filter/decant to remove insoluble
material, concentrate by boiling/evaporation and finally allow to cool, hopefully forming crystals of the sulfate salt of isophorone diamine.
[Edited on 21-10-2013 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Okay, but then I might as well destill off most of the water from the mix I currently have, and see whether C10H22N2.H2SO4 will crystallise from the
solution on cooling, correct?
I will try tonight, if time permits.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bezaleel  |
2. Let stand, so that the benzyl alcohol will largely separate.
3. Decant, keeping the colourless watery part. |
I hope that you kept the benzyl alcohol/diamine phase?! 
(As I mentioned a month ago: adding benzyl alcohol/diamine to a solution of oxalic acid in acetone would precipitate the diamine as a salt).
[Edited on 22-10-2013 by sonogashira]
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Quote: Originally posted by Bezaleel  | Okay, but then I might as well destill off most of the water from the mix I currently have, and see whether C10H22N2.H2SO4 will crystallise from the
solution on cooling, correct?
I will try tonight, if time permits. |
You could, and it may work. But you are assuming that the isophorane diamine dissolved in the unacidified water phase. If you get no yield, it
possibly just remained in the benzyl alcohol phase. The fact that its miscible with water does not necessarily mean that you were able to extract much
of it from the other phase in that way.
Like sonogashira says, let's hope you kept the benzyl alcohol phase.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Almost everything, because you failed already in the first step. Please read on the concept of liquid-liquid partitioning.
Additionally, I don't understand what you mean by the "idea being that the sulfate of isophorone diamine will crystallise from the solution." I doubt
the sulfate is insoluble in water. What is the reference for that?
| Quote: | | Question 2: I noticed that the yellow layer, supposedly containing most of the benzyl alcohol, dissolved very well in blue spirits (which turned a bit
greenish). Is it worth trying to add H2SO4 to an ethanolic solution of the hardener, to separate out the sulfate of isophorone diamine? Is it okay to
use ketonated alcohol as the solvent, instead of pure alcohol (compatibility wigh reaction)? |
Don't mess with H2SO4 on a mixture of benzyl alcohol and a diamine. You would need to use exactly a stoichiometric amount while you don't have the
assay of your mixture. You would also need to check the literature, if the sulfate is crystalline and insoluble in ethanol.
I can't help wandering why do you go round and round instead of doing it properly. Why don't you just do a simple acid/base extraction? Or just make a
salt like sonogashira suggested? There really is no need to reinvent the wheel, especially if you don't have the knowledge necessary. Believe me, you
will only end up frustrated.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|