| Pages:
1
..
48
49
50
51
52
..
76 |
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Quote: Originally posted by Velzee  |
He's back! Welcome back!
Yes, as DraconicAcid said, I made an attempt at potassium ferrioxalate, but I forgot to account for the stoichometry when I replace KOH with K2CO3,
thus excess contaminates remained. |
Lol thanks Velzee  . .
Actually I still check sm from time to time. Summer holidays are just too busy  . .
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Hopefully some Vanadium(II)

|
|
|
Arg0nAddict
Hazard to Self
 
Posts: 54
Registered: 8-6-2016
Location: PNW
Member Is Offline
Mood: Mercuric
|
|
This was an experiment for storing sodium short term without an oxide layer and to get pictures for ebay ads.
30 grams of Sodium was made molten inside a bath of mineral oil, I believe paraffin oil, and then injected into room temperature, 70°F (~21°C), oil
using an all glass 10mL syringe.
I have been trying several different techniques to get uniform sizing and the middle section, where it looks the best, is what I described above. The
bottom 1/4 of the vial I used cold oil 50°F (10°C). The top 1/4 of the jar was hot oil, 100°F (~38°C).
**SAFETY NOTES**
I learned the hard way that molten sodium will fall out of the syringe as soon as it points just 1° below 180° luckily it bounced off my finger and
didn't do more damage. In the gallery you'll see the Pea size blister on the tip of my pinky finger.
Clean sodium catches fire much easier, especially when shiny and molten.
-------------------
Beaded Sodium Gallery ~ 8 more pics
-------------------


Beaded Sodium Gallery ~ 8 more pics
[Edited on 2-9-2016 by Arg0nAddict]
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Absolutely beautiful Arg0nAddict!
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Gorgeous.
I think the variable size adds to the aesthetic. And probably makes it more usable -- if you are using it for more than just a display.
|
|
|
Arg0nAddict
Hazard to Self
 
Posts: 54
Registered: 8-6-2016
Location: PNW
Member Is Offline
Mood: Mercuric
|
|
https://www.youtube.com/watch?v=iRTDf4m6V0k
Not a pretty photo but it is my adaptation of the dancing sodium reaction. The music is important as I think you all will enjoy it.
|
|
|
Arg0nAddict
Hazard to Self
 
Posts: 54
Registered: 8-6-2016
Location: PNW
Member Is Offline
Mood: Mercuric
|
|
Here's some more sodium, which is best?



|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Third one IMO, but they are all pretty pictures  . .
|
|
|
Velzee
Hazard to Others
  
Posts: 379
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
I made this one as a birthday gift for a friend; it is a two week old potassium ferricyanide crystal.
 
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
beautiful!
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
That is mighty impressive, nice! What an awesome gift.
[Edited on 11-9-2016 by violet sin]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
How did you make it? Slow evaporation with a seed crystal, or something like that?
|
|
|
Fidelmios
Hazard to Others
  
Posts: 104
Registered: 28-4-2016
Location: Witty Chemistry Joke
Member Is Offline
Mood: No Mood
|
|
Yeah my current potassium ferricyanide crystals aren't coming out yet. It's like they're neither growing or dissolving.
|
|
|
Velzee
Hazard to Others
  
Posts: 379
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Yep!
@Fidelmios how long have you had the solution left out?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
So we took a thermal image of a cup of coffee and saw these darker spots which always move in thise kind of net shapes on the surface.
I changed the colors so it becomes easier to see and added some temperatures so you can see the difference. For some reason these spots are around
3-4°C cooler than the rest of the coffee.
I think it might be the oils which are hydrophobic and collect on the surface but as oils dont evaporate that easily the stay longer on the surface
and can therefore cool off a bit which makes these spots colder. At first I thought it was only a minor temperature difference but when I looked into
the data I saw that there is a surprising temperature difference. Any idea ? I waited till the surface was perfectly still and most of the boiling and
fuming was over before I took the image.
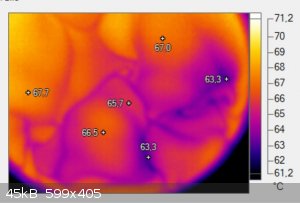
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  | So we took a thermal image of a cup of coffee and saw these darker spots which always move in thise kind of net shapes on the surface.
I changed the colors so it becomes easier to see and added some temperatures so you can see the difference. For some reason these spots are around
3-4°C cooler than the rest of the coffee.
I think it might be the oils which are hydrophobic and collect on the surface but as oils dont evaporate that easily the stay longer on the surface
and can therefore cool off a bit which makes these spots colder. At first I thought it was only a minor temperature difference but when I looked into
the data I saw that there is a surprising temperature difference. Any idea ? I waited till the surface was perfectly still and most of the boiling and
fuming was over before I took the image.
|
They look like the tops of convection cells.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by mayko  | These lovely blue mushrooms pop up around this time of year. Turns out, they also bruise a lovely green color!
Incidentally, does anyone have protips on preserving mushrooms? I tried putting this one in a closed container with some CaCl2 but it just got slimy
and grungy...
|
It's a breakdown product of the psilocybin. Sun dry them, then freeze. (Have experience this area; they're worth $240/OZ in California. Idiots)
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Maybe you can repeat the experiment after adding a drop of soap? That could test your hypothesis.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by arkoma  | Quote: Originally posted by mayko  | These lovely blue mushrooms pop up around this time of year. Turns out, they also bruise a lovely green color!
Incidentally, does anyone have protips on preserving mushrooms? I tried putting this one in a closed container with some CaCl2 but it just got slimy
and grungy...
|
It's a breakdown product of the psilocybin. |
???
Wikipedia says the blue pigment is an azulene derivative:

https://en.wikipedia.org/wiki/Lactarius_indigo#Chemical_comp...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
generally only Psilocybe or Stropharia stain that weird blue-green colour when bruised. Mmm, This genus too. I have a dab of *ahem* up close and personal experience with these particular fungi.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by arkoma  | | protips on preserving mushrooms? I tried putting this one in a closed container with some CaCl2 but it just got slimy and grungy...
|
Treat them like anything you want to keep oxygen and water away from.
Sun-dry first (if you got Lotsa Sun) or/then bag with a jar of a water sucker.
E.g. NaOH, K3PO4, conc sulph acid, shop-bought rice, some table salt etc.
|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
My new crystal specimens:
Xylitol

Saccharin (sodium salt)

Saccharin is not sold anywhere nearby me, so I managed to extract it from the cyclamate-based sweetener (usually containing 90% of Na cyclamate and
10% of saccharin). Compound for this photo was extracted from 210g of sweetener, that gave me 18g of saccharin (not a bad yield I think). As a
byproduct, I now have 180g of recrystallized sodium cyclamate, and no idea what to do with it.
Potassium zinc sulfate K2Zn(SO4)2*6H2O

|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow, really amazing crystals Dmishin!
Are all of these crystals products of slow evaporation?
And if yes, how long did it take to grow them?
Thanks for answer
|
|
|
Dmishin
Harmless

Posts: 32
Registered: 5-10-2015
Member Is Offline
Mood: No Mood
|
|
Yes, all grown by evaporation. Growth time is from 2 to 4 weeks, depending on sample. By the way, due to tilt shift effect, xylitol crystal seems
small, but it is actually 4cm wide.
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Hmmm, I've never grown xylitol crystals. I ought to try it  . .
|
|
|
| Pages:
1
..
48
49
50
51
52
..
76 |