| Pages:
1
..
24
25
26
27
28
..
78 |
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Reporting back.
I mixed up a 62/38 blend of KNO3 and powdered sugar. Actually, a lot more than I intended since I inadvertently made a 38/62 blend and had to add
some more nitrate.
A test burned nice and feisty. My rocket engine design needs a bit more work. Attempting to keep things small scale, I packed the material inside
short sections of drinking straw. But, I gave little thought to the nozzle, had no decent fuse and with the narrow diameter, I did not drill a core
hole. Lots of smoke and fizzing but little in the way of flight. Turns out that Mr 4 wasn't that interested this time around anyway. Miss 7 thought
it was great. We had a good time adding various agents to the fuel mix to achieve different kinds of sparkles and colour. Ti powder was definitely
the most impressive although Zn powder was pretty cool too. (I think however the Zn was too fine.) Strontium carbonate failed to produce the red I
was hoping for and CuO didn't produce a particularly vibrant colour either.
I have quite a bit left and will attempt a casting some time -- probably with some titanium added.
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
Guys...firework cake:12 cardboard tubes with 6 40mm shoots/each tube.How much black powder would take take(for lift,give me an example)?
[Edited on 15-10-2015 by Tabun]
|
|
|
Gargamel
Hazard to Others
  
Posts: 166
Registered: 9-3-2013
Member Is Offline
Mood: No Mood
|
|
| Quote: |
12 cardboard tubes with 6 40mm shoots/each tube |
That would mean some kind of roman candle construction?
It's hard to answer your question, because there are to many uncertain factors involved.
In a "normal" cake I would use something like 15-20% of your bombette weight, assuming
-your BP is of good quality
-and your bombettes fit in the tube with very little play.
Advice:
don't start with to complicated devices. Try to create a single shot with one bombette first and dial it in properly. That does not sound to hard, but
just try it, if your new to pyro it will take you some time until it works.
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
I don't know if it's called the same in english,I found that cake translation after looking at some videos.We call it "Firework battery" here.An array
of tubes with shells launching one after another.
By the way...can you tell me if H3 can be trusted?Not for lift,not for power,not for shock/thermal sensitivity but for how much it can stay safe and
good if nothing interacts with it(like sulfur).I can't find too many videos or info on it.
[Edited on 15-10-2015 by Tabun]
|
|
|
Gargamel
Hazard to Others
  
Posts: 166
Registered: 9-3-2013
Member Is Offline
Mood: No Mood
|
|
We call it "battery" too. Like a battery of guns. "Cake" is American.
| Quote: |
An array of tubes with shells launching one after another. |
Yes. But usually there is only one shot in every barrel.
| Quote: |
can you tell me if H3 can be trusted? |
Yes it can. If one knows how to handle it. H3 can be trusted, inexperienced users usually not 
It could be used for lift in small calibres or to break shells.
But anything you have in mind can be done with BP alone, without the chlorate related troubles. It's hard not to have any sulphur contact.
Or your bombettes have nitrate and metal in them -> ammonia -> ammonium chlorate.
Or your cake is a used salvaged one and has acidic residues in the tubes...
ect. ect.
If you want to do some serous pyro, you cannot avoid to learn how to make proper BP. I would suggest not to take H3 as a shortcut.
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
I wasn't thinking about using H3 for lift.I know BP doesn't work too well for firecrackers so I was thinking about the H3.It comes in contact only
with the tube and the fuse...
[Edited on 16-10-2015 by Tabun]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by James Ikanov  | So, I have what might be one of the dumbest questions I've asked in a while...... Does anyone know of any synthesis or inquiry related to the nitrites
(not nitro, NO groups) of toluene or other similar compounds (xylene, benzene) ? The closest I could find was a paper written in the late sixties in
london that was using it to figure out the stereospecificity of xylene.
As an aside, I was wondering if anyone had any solid information on the properties of nitroxylene (20ml of Xylene). I recently made some using the
commercial grade stuff (at least, I think) through solution of sulfuric acid(50ml)/KNO3(15g, way way too much). After I added the xylene it stratified
rather quickly, even when stirred. I then poured it into a separatory funnel. It stayed separated into layers and I drained off the acid into some
water very slowly (what I think is nitroxylene stuck towards the top), and was left with a milky white fluid which I poured off into an amber sample
jar and refrigerated.
I am very interested in seeing if I can find anyone who has sample to compare to or some other information on the topic. Even if it's a very wimpy
explosive I'm still somewhat interested just because of how incredibly easy it was to make this small-ish sample. 
I've exhausted my ability to find information on either at this point, I think. |
If you are refering to Ar-N=O compounds then those are nitroso-aromatics and not nitrites what would be Ar-O-N=O.
Nitrites of aromatic compounds would be mixed anhydrides of phenic acid (phenol) and nitrous acid...
Ar-O-H + HO-N=O <==--> Ar-O-N=O + H2O
and as such unstable usually transfering the nitrosonium cation in para (or in ortho if the para position is not free) of the phenate anion...
Ar-O-N=O <--==> Ar-O(-) + N=O(+) --> (-)O-Ar-N=O + H(+) --> HO-Ar-N=O (nitrosophenol)
Phenols do react very easily with nitrous acid, nitrous esters or nitrosonium generators like Cl-N=O (nitrosyl chloride).
Alkylbenzene need more activation to introduce a nitrosonium so maybe mesitylene (1,3,5-Ar(CH3)3) will make a nitrosomesitylene.
I doubt 1,3-xylene (m-xylene) will do it (and this would be the more favourable one since 1,2-xylene (o-xylene) or 1,4-xylene (p-xylene) will have
counteractive activation from their methyls groups.
You used commercial grade xylene and this contains normaly all three flavors (ortho, meta and para xylene) thus you already start with an horrible
array of possible mononitrocompounds (2 for ortho, 3 for meta (with two major and one minor) and 1 for para.
This may account for your oily stuff (each compound will depress the melting point of the other) even if you succeeded in full polynitration of
xylenes.
Also the methyl groups tends to lower the mp of the poly-nitroaromatics and the density...they also lead to worst OB...yielding poor TNT équivalents.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Q1)Can HEs like ETN or RDX e.t.c. be used as anion or cation ? Like dinitroguanidine (ammonium dinitroguanidine / dinitroguanidine nitrate) ?
Q2) Can guanidine and glyoxal react with the same way as urea and glyoxal does to form glycouryl ? but replace the two C=O bonds with N=O bonds
[Edited on 27-10-2015 by underground]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by underground  | Q1)Can HEs like ETN or RDX e.t.c. be used as anion or cation ? Like dinitroguanidine (ammonium dinitroguanidine / dinitroguanidine nitrate) ?
Q2) Can guanidine and glyoxal react with the same way as urea and glyoxal does to form glycouryl ? but replace the two C=O bonds with N=O bonds
[Edited on 27-10-2015 by underground] |
If you look at RDX, its a stabalized ring compound and does not have acidic H atoms, therefore it can not be a anion..
it also does not have any groups to protonate so RDX can not be a cation too.
Erythritol is a sugar and same story..
[Edited on 28-10-2015 by DubaiAmateurRocketry]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
did anyone succeed in synthesis of triaminotrinitrobenzene ?
if yes, what was your setup and the synthesis path you used?
[Edited on 28-10-2015 by ecos]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by underground  |
Q1)Can HEs like ETN or RDX e.t.c. be used as anion or cation ? Like dinitroguanidine (ammonium dinitroguanidine / dinitroguanidine nitrate) ?
Q2) Can guanidine and glyoxal react with the same way as urea and glyoxal does to form glycouryl ? but replace the two C=O bonds with N=O bonds
|
Q1) Not per se, but one may imagine some groupings added to such HE strutures that may display acidic or basic properties to help getting such ionic
character favorizing the making of salts
-CO2H (carboxyl)
-CH2-NO2 (nitromethyl)
-CH(-N=O)-NO2 (nitroso-nitromethyl)
-CH(NO2)2 (dinitromethyl)
-NH-Ar(NO2)3 (trinitroanilidyl)
-SO3H
-CO-NH-CO-
-CH(C#N)2
...
-NH2
-NH-CH3
-N(CH3)2
-C(=NH)-NH2
-NH-C(=NH)-NH2
-idem supra but with NH2-NH2 dérivatives
...
-Tetrazolyl group
...
There are plenty of possibilities, only limit is your imagination and the stability (chemical but also storage and sensitivity) of the explosophoric
groups towards the added acidic or basic group.
Example based on the building block "(O2N)3C-CH2-":
(O2N)3C-CH2-CO2H + H2N-NH-CH2-C(NO2)3 --> (O2N)3C-CH2-CO2H3N-NH-CH2-C(NO2)3
Q2) It is actually C=NH groups that replace the two C=O groups not N=O ones!
Maybe that guanidine and glyoxal tends to form a 3D polymeric material instead of the wished polycyclic or caged molecule...
The 3 NH2 in guanidine may indeed react equivalently.
[Edited on 29-10-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yes i mean C=NH groups that replace the two C=O groups
I want to avoid the hydrolysis duo to C=O group.
Overall looks a really powerfull and easy to make HE for it's performance
|
|
|
dieglegold
Harmless

Posts: 15
Registered: 17-10-2015
Member Is Offline
Mood: No Mood
|
|
Touch powder
I have seen the term "touch powder" mentioned on this blog site. What is touch powder?
|
|
|
Bert
|
Threads Merged
2-11-2015 at 10:58 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Probably nitrogen triiodide.
Unlikely to ever see it as a powder, at least not for long.
Explodes at the slightest energy input.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Armstrong's mix, made with finely divided and good quality red phosphorus and potassium chlorate will deflagrate or detonate very easily. A gentle
poke from a wooden dowel or popsicle stick is often enough for a surprise. This mix is a powder and would likely fit your description.
If you decide to mix some, use only very small amounts. Like 100 mg or less. Blending the reactants often causes them to ignite, even when just
diapering them in paper, I have heard. Larger batches will cause bodily harm to the mixer, especially if there is enough powder being mixed, or the
batch is partially confined enough to detonate.
[Edited on 2-11-2015 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
dieglegold
Harmless

Posts: 15
Registered: 17-10-2015
Member Is Offline
Mood: No Mood
|
|
I have made nitrogen triiodide ever since I was a kid. It is a wickedly dangerous compound, and a lot of fun.
I have purchased, from a magic supply house, red phosphorus and a white powder, most like potassium perchlorate (or chlorate?) When a bit of red
phosphorus is smeared on the forefinger and a bit of the white powder is smeared on the thumb, one can make a loud bang by snapping the fingers. (No,
I don't do this very often!)
|
|
|
dangerous amateur
Hazard to Others
  
Posts: 144
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
PETN, ETN ect. neutralisation, carbonate vs. bicarbonate
Hi Guys,
I wonder what works better for neutralisation purposes.
Last time I used sodium carbonate for neutralisation of my PETN before recrystalising.
But the sodium carbonate doesn't dissolve very well and leaves behind a milky tint in the water, which is hard to wash of the PETN.
Would sodium bicarbonate be more suitable for the job?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by dieglegold  | | I have purchased, from a magic supply house, red phosphorus and a white powder, most like potassium perchlorate (or chlorate?) When a bit of red
phosphorus is smeared on the forefinger and a bit of the white powder is smeared on the thumb, one can make a loud bang by snapping the fingers. (No,
I don't do this very often!) |
How long ago was such a combination of chemicals sold, and in what COUNTRY?!
And how did your fingers feel after...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Explosive colophony rosin?!
One of my crew is a violist, sent me this:
http://www.theviolinchannel.com/violin-rosin-confiscated-chi...
Chinese customs inspectors seem to think rosin used on bows of violins & etc. is dangerously explosive?!
It certainly will BURN- But outside of some rather old pyrotechnic star mixes and some equally old fashioned salute mixtures with Potassium chlorate,
what could be their justification?
| Quote: |
Word is circulating today that a number of string players have had their rosin confiscated, from their cases at Chinese airports, in recent weeks –
with airport officials claiming it is a potential explosive.
A Hong Kong newspaper, Mingpao has reported cellist Trey Lee’s rosin was seized when departing Shanghai International airport earlier this month –
with lengthy claims to its potential security risk.
British violin virtuoso Daniel Hope has also indicated, via social media that he too experienced a similar confrontational incident when departing
Shanghai in recent weeks.
|
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
ALICE as EM
Hi All,
I found this table in Patent US 20120216926 A1 It is about new propellent that consists of Al + ice water or hydrated compound.
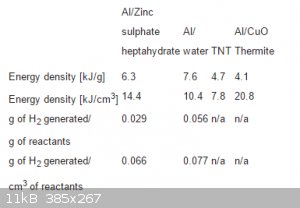
The numbers surprised me ! does this mean that ALICE can be a better explosive than TNT ? what would be the expected VoD ?
[Edited on 7-11-2015 by ecos]
|
|
|
dangerous amateur
Hazard to Others
  
Posts: 144
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
How often do you recrystallize your ETN?
Yes I know, until it's neutral.
But how many steps does it usually take for you to get it 100% neutral?
I usually wash it with sodium carbonate solution first, then with additional water.
Then I recristallize it from ethanol.
I cannot find any acid detectable with ph strips.
However, would you recristalize a second time?
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Depends on how you recrystallize it, if you used a fair excess of alcohol with the dissolve/crash method you should be fine.
Two or three is better than one but in my experience it's usually pretty storage stable after just one with that said I wouldn't be making cast
charges with it.
[Edited on 13-11-2015 by OneEyedPyro]
|
|
|
dangerous amateur
Hazard to Others
  
Posts: 144
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Thanks.
No melting is intended.
Do you use something to neutralize the residual acid trapped in the cristals during the recristallization process, or do you just rely on the alcohol
to dillute it?
Because I found both sodium carbonate and bicarbonate wont dissolve in ethanol.
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Like I said, it depends on the type of recrystallization you perform.
If you heat your solvent, dissolve ETN then cool to recrystallize you should have quite pure ETN.
If you dissolve ETN then dump it into cold water to recystallize you can always add a carbonate/bicarb or a stabilizer such as urea to the water.
|
|
|
Bert
|
Threads Merged
14-11-2015 at 18:40 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | Hi All,
I found this table in Patent US 20120216926 A1 It is about new propellent that consists of Al + ice water or hydrated compound.
The numbers surprised me ! does this mean that ALICE can be a better explosive than TNT ? what would be the expected VoD ?
[Edited on 7-11-2015 by ecos] |
If you look at the meaning of energy density, you will soon realize that many things are better than TNT or usual HE.
Typical examples are compressed hydrogen or pure carbon dust...also comes to mind superfuels like dicyanoacetylen, dicyanohexadiyne or boranes.
But those doesn't display explosophoric properties alone, you need an oxydizer.
What is good for a propellant application is not always good for blasting/cuting materials.
The Al-ICE type of propellants may deflagate or detonate only under very specific set of parameters, if it succeeds to go to detonation one should
expect something between 5-3 km/s VOD
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
24
25
26
27
28
..
78 |