| Pages:
1
..
49
50
51
52
53
..
78 |
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Quote: Originally posted by PHILOU Zrealone  |
[/rquote]
Ingenious.
A flash powder Al + KMnO4
+
Al/ETN D2D explosive
+
ETN a high detonating explosive

I'm more than eager to see your study of it and stability.
No moisture, and good quality Al powder --> no risk. |
Well, thanks for the compliment. KMnO4 is free available against NH4ClO4. AP working also, described before estimate year. But making NPED with
trivial catalysator as is cheap permanganate + aluminium is almost beyond belief. Huhaha...Simply...flash powder....
"Let us do things as simple as possible. But not simpler."......Albert Einstein......
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
H2O2
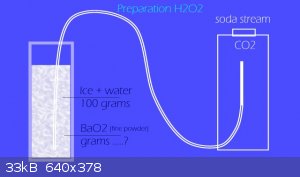
I have a short question for experts. Is possible counting, how much is need of BaO2 for preparation 30 - 35% concentration H2O2 ? For example as base
can be 100g water, respectively the crushed ice and zero cold water. Carbon dioxide is available in any amount, of course. Can be bubbled slowly and
by long time.
According stoichiometry should by : 100g H2O + 940g BaO2 + 244g CO2 = 188,8 H2O2 + 1095g BaCO3 (insoluble). But it is for absolut 100% H2O2. Which is
nonsense. Is thus possible use (for 30% H2O2) 313g BaO2 ?
It seems, that it will be expensive thick slurry and not hydrogen peroxide.......... ........Thanks...... ........Thanks...... .......LL .......LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
hnto
Harmless

Posts: 4
Registered: 11-1-2017
Member Is Offline
Mood: No Mood
|
|
Question on stirbars and a concave flask (convex on the inside):
I have a flask that I picked up as a part of a bunch of different things. This one has a concave bottom (causing a dome of glass inside the flask).
It's a great flask, but I can't use it because my magnetic stirbars won't stay on the top of the dome. Odd that the flask is designed this way and
wondering what kind of stirbar one might use with this kind of flask?
I don't have a picture I can post of it at the moment, but it is basically like a round-bottom flask with the bottom of it punched in so-to-speak.
Thanks so much to anyone who might be able to point me in the right direction of buying something that would work for this flask!
[Edited on 6-2-2017 by hnto]
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
I can't answer your question, but I did have another one you reminded me of.... Have you ever tried fitting an N2O cylinder for whipped cream making
into your soda stream? 
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
hnto
Harmless

Posts: 4
Registered: 11-1-2017
Member Is Offline
Mood: No Mood
|
|
Oh, I think I've found something that may work. Would love to know if anyone has experience with these:
Spinbar® Circulas™ (Dumbbell-shaped)
This magnet stirring bars provide strong turbulence at relatively low speeds, offer reduces surface contact and have excellent centering
characteristics, particularly in vessels with convex bottoms. Ideal for all “problem” liquids, highly viscous mixtures, metals particles in fluid.
All sizes have solid PTFE end disks 20 mm (.79”) in diameter, and bar diameter is 8 mm (.315”)
Or
http://www.bdl-cee.com/home/magnetic-stirring-bars-dumbbell-...
I think the dumbbell shape would keep it on top of the dome of glass if I have the right length/size of dumbbell stirbar.
|
|
|
Chlorine
Hazard to Self
 
Posts: 56
Registered: 26-11-2016
Location: Maine, USA
Member Is Offline
Mood: Brominated
|
|
Quote: Originally posted by hnto  | Oh, I think I've found something that may work. Would love to know if anyone has experience with these:
Spinbar® Circulas™ (Dumbbell-shaped)
This magnet stirring bars provide strong turbulence at relatively low speeds, offer reduces surface contact and have excellent centering
characteristics, particularly in vessels with convex bottoms. Ideal for all “problem” liquids, highly viscous mixtures, metals particles in fluid.
All sizes have solid PTFE end disks 20 mm (.79”) in diameter, and bar diameter is 8 mm (.315”)
Or
http://www.bdl-cee.com/home/magnetic-stirring-bars-dumbbell-...
I think the dumbbell shape would keep it on top of the dome of glass if I have the right length/size of dumbbell stirbar. |
I haven't used this exact stir bar, but i have tried quite a few of the "fancy shaped" ones. Overall there isn't much of a difference, i've always
stick with the atypical rod shaped stir bar.
Regards,
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  |
I have a short question for experts. Is possible counting, how much is need of BaO2 for preparation 30 - 35% concentration H2O2 ? For example as base
can be 100g water, respectively the crushed ice and zero cold water. Carbon dioxide is available in any amount, of course. Can be bubbled slowly and
by long time.
According stoichiometry should by : 100g H2O + 940g BaO2 + 244g CO2 = 188,8 H2O2 + 1095g BaCO3 (insoluble). But it is for absolut 100% H2O2. Which is
nonsense. Is thus possible use (for 30% H2O2) 313g BaO2 ?
It seems, that it will be expensive thick slurry and not hydrogen peroxide.......... ........Thanks...... ........Thanks...... .......LL .......LL |
Yes should work...
Or you increase the H2O volume (or weight) by 3 times --> 300g
Or you reduce the amount of BaO2 by a factor 3 --> 313,3g
And for BaO/BaO2 and CO2 recylcing...
Use acetic acid    
BaCO3 + 2 HO2C-CH3 --> Ba(O2C-CH3)2 + H2CO3
H2CO3 <---===> H2O + CO2(g)
Ba(O2C-CH3)2 --dry heat--> BaO + CH3-CO-CH3 + CO2(g)
BaO + O2 --dry heat--> BaO2
So as a bonus you get aceton (caution flamable so good condenser needed).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
H2O2
Well, if I understand, is possible use only 104g BaO2 + 100g ice/water and bubble. After making decantation BaCO3. Cooling again on ice/water (now cca
10% concentration of H2O2) and added again 104g BaO2. Again bubble CO2 and decantation BaCO3. Now we have 20% H2O2. And all step repeat still one.
Thanks, Philou... ...LL ...LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
nitroguanidine is a stable explosive with a high V det (>7000m/s) with the chemical formula of CH4N4O2
guanidine nitrate has the chemical formula of CH6N4O3, so we are able to create one more H2O... but from my understanding guanidine nitrate just
produces gas for airbargs and monopropellents... why is it not explosive??
From looking at it's chem formula I would think it would be more explosive...? What part of chemistry do I need to rigorously study to know this
without experimentation...?
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
Cursory googling suggests that Nitroguinadine is one molecule whereas guinadine nitrate is a bit more like... I'm not sure of how I would describe it
other than a nitrate ion hanging out very closely to a guinadine molecule?
Wikipedia has a similar sentiment on the matter....
https://en.wikipedia.org/wiki/Guanidine_nitrate
https://en.wikipedia.org/wiki/Nitroguanidine
Wikipedia also says that nitroguanidine is used as an airbag propellant as well.
Perhaps this short excerpt will enlighten you, I don't completely understand the mechanism it describes but it seems to back up what I have stated:
"Nitroguanidine is produced commercially by a two step process starting with the reaction of calcium cyanamide with ammonium nitrate. Via the
intermediacy of biguanidine, this ammonolysis step affords the salt guanidinium nitrate. In the second step, the nitrate salt is treated with sulfuric
acid, a process that dehydrates the salt and forms the N-N bond.[1]"
[C(NH2)3]NO3 → (NH2)2CNNO2 + H2O
On an unrelated note, I'm very curious, has anyone ever attempted to make an EBW capacitor bank out of a set of leyden jars? it seems like an
extremely cost effective methodology given the extremely high voltage capacity for something so easily made out of such common materials....
[Edited on 9-2-2017 by James Ikanov]
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | nitroguanidine is a stable explosive with a high V det (>7000m/s) with the chemical formula of CH4N4O2
guanidine nitrate has the chemical formula of CH6N4O3, so we are able to create one more H2O... but from my understanding guanidine nitrate just
produces gas for airbargs and monopropellents... why is it not explosive??
From looking at it's chem formula I would think it would be more explosive...? What part of chemistry do I need to rigorously study to know this
without experimentation...? |
Both are very unsensitive explosives (to friction and shock) with comparable volume of detonation gases (1098L/kg for GN and 1075L/kg for NQ)-->
they need strong confinement for detonation.
Differences are to be found into: (data from Rudolf-Meyer's Explosives)
-low comparable lead block test expansion (GN = 240 cm3/10g vs 305/10g for NQ).
-low specific energy (964 kJ/kg for NQ and 750 kJ/kg for GN)
-low heat of explosion (3220 kJ/kg for NQ and 2604 for GN (H2O(l))
So those are cold exploding stuffs (the heat of explosion is about 50% less than NM or NG certainly linked to the very negative heat of formation,
poor OB and H2O generation)...lead block test shows that they are not very powerful (kind of 50% of EGDN) and at best perform like would unperfectly
balanced dinitroaromatic explosives.
This makes them good for corrosion free propellant and gunpowder applications or air bags..."cold burning"
NQ is slightly better as a detonating explosive because it contains less water and reach thus a higher explosion temperature but also because it is
slightly denser than GN...
Water tends to increase specific impulse for rocketry applications but is detrimental to brisance because it reduces the heat of the detonation
products (gases).
[Edited on 10-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Certain types of German dark and Indian Blackhead Al powder are 2-5 microns. Is this the thickness of the flake, or the length.
For example, a russian Al powder named PAP-2 has an average thickness of .2-.5 microns but the length of the flake is 20-30 microns... so would this
be smaller or larger than the above powders??
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Both smaller and larger ;-)
In fact the active surface (volumic surface) is much bigger for the PAP-2...for sure both powders should provide very different properties to pyro
mixes...oxyd layer will play a bigger role into the PAP-2 aswel.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
PAP powders are coated with a very persistant wax like substance that prevents them from oxidizing in air and even water for that matter. A local
aerated concrete block producer in our vincinity uses PAP Al powders as gas generation media in their AAC block mixture and they used a separate
washing step in alkaline media to rid the powder of the protective coating and get the most out of it.
Exact science is a figment of imagination.......
|
|
|
JohnDoe13
Harmless

Posts: 21
Registered: 3-2-2017
Member Is Offline
Mood: No Mood
|
|
The waxy coating is Stearin. It can be easily removed with acetone, but these types of aluminum powders are very reactive even with the stearin
coating.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Well I want some, but had trouble finding sources, does any of you gentleman know where I can get this? (websites) I am ok ordering it form a
different country of orgin!
All of the nano Al I am finding (100nm) is priced at about 3-5$ a gram shipped, wow! I would definitely like to try the PAP first. researchers seem
to have great success with it.
[Edited on 16-2-2017 by MineMan]
|
|
|
JohnDoe13
Harmless

Posts: 21
Registered: 3-2-2017
Member Is Offline
Mood: No Mood
|
|
Search for paint grade aluminum powder. Most of the time it's flake aluminum.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Screening Fine Chemicals.
Hello All,
To follow Berts advice I am trying to nail the particle size for flash powder. I am trying to screen potassium perchlorate ground in a mortar and
pestal. I can get my particles through the 250 and 120um screen. But I cannot get particles through the 53um, 25um, and 10um screen... I think
because of clumping.
What Can I do, advice?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by MineMan  | Screening Fine Chemicals.
Hello All,
To follow Berts advice I am trying to nail the particle size for flash powder. I am trying to screen potassium perchlorate ground in a mortar and
pestal. I can get my particles through the 250 and 120um screen. But I cannot get particles through the 53um, 25um, and 10um screen... I think
because of clumping.
What Can I do, advice? |
Maybe anti-caking agent...
Like for kitchen/table salt...for example using Potassium feri- or fero- cyanide...easily revealed by formation of blue cyan colour (lappis lazulis)
upon contact with FeCl2...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
There is a specific product for this purpose, it is a type of fumed silica and you can sometimes buy it off ebay but I can't remember the trade name,
a small amount is added before milling. Also you need to make sure your perchlorate is perfectly dry (use an oven at 110 C; before mixing!) before you
grind it.
Edit: is available under the name Carb-o-sil in the UK from various ebay suppliers.
[Edited on 25-2-2017 by Boffis]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by MineMan  | Screening Fine Chemicals.
Hello All,
To follow Berts advice I am trying to nail the particle size for flash powder. I am trying to screen potassium perchlorate ground in a mortar and
pestal. I can get my particles through the 250 and 120um screen. But I cannot get particles through the 53um, 25um, and 10um screen... I think
because of clumping.
What Can I do, advice? |
Maybe anti-caking agent...
Like for kitchen/table salt...for example using Potassium feri- or fero- cyanide...easily revealed by formation of blue cyan colour (lappis lazulis)
upon contact with FeCl2... |
The use of such fero or feri cyanides is also a good way to increase energy output (see Dornier deflagro-detonating mix of such cyanides with
chlorates...)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I don't understand, does the fero and feri cynaides help with screening??
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Ammonium Perchlorate Decomposition Temperature.
Hello All,
I was upset to find that the decomposition temperature of ammonium perchlorate is only 130 degrees C. Is this correct? How could it be used for
missiles and other military applications if it decomposes at such a low temperature??
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
In wikipedia is described 200 - 240 C for decomposition AP. 130 is nonsense. Maybe from homemade reactor if is contamined NaClO4. His melting point is
130 C when evaporate, respectively his decompose on pure dry NaClO4 + H2O steam. Dr.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If you problem is really clumping (= caking) thus anti-caking agent should solve this...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
49
50
51
52
53
..
78 |