| Pages:
1
..
62
63
64
65
66
..
78 |
c1der
Harmless

Posts: 2
Registered: 29-4-2018
Member Is Offline
Mood: No Mood
|
|
Determination of 2,4,6-trinitrophenol or 2,4-dinitrophenol
Is there a "home chemistry" way to determine if my final product from ASA sulfonation and nitration is 2,4,6-trinitrophenol and not 2,4-dinitrophenol?
|
|
|
urea1990
Harmless

Posts: 13
Registered: 20-3-2017
Member Is Offline
Mood: No Mood
|
|
Wearing proper respiratory protection, heat a minute amount of the product to 125C. Make sure it doesn't reach 150C (picric acid's flash point). If
your product is dinitrophenol, it'll boil off; picric acid will just melt.
|
|
|
c1der
Harmless

Posts: 2
Registered: 29-4-2018
Member Is Offline
Mood: No Mood
|
|
Sorry, but I don't have precise temperature control. Is there any other way?
[Edited on 30-4-2018 by c1der]
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
By the way KCLO3 flash powders are way stronger than KCLO4. I made a comparison video i will upload it soon
**edit
https://youtu.be/1tdmSpzAqpg
[Edited on 30-4-2018 by feedme94]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
You probably mean to say that the chlorate mixtures you tested appear to be more brissant, from dammage done to your witness plates (pop cans).
Stronger implies more total energy released- per weight? Per volume?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
You are right , my english are not that good. Chlorate mixtures are more brissant and also found that sulfur increase the brisance in all mixtures i
tested. I also think that KCLO3 70% / AL 30% mix was the loudest.
[Edited on 30-4-2018 by feedme94]
|
|
|
Bert
|
Threads Merged
30-4-2018 at 12:14 |
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
While looking for ways to make fine aluminum powder, I came across this Instructables page online, by Dornier 335A. (http://www.instructables.com/id/How-to-make-super-fine-powde...) I also found this SM post, and a few others (http://www.sciencemadness.org/talk/viewthread.php?tid=21516&...) Unfortunately, the instruction is in a YouTube video, and since Dornier's channel
was taken down, I can't watch the video. I was wondering if anyone here can explain this process. If it's too complicated to explain, ignore this and
don't worry about it. I'll stick with the ball mill, or something. It sounds like you grind the metal between a steel plate and a steel bar? Is there
any particular pointers for making it fine? Thank you.
Put that in your pipe and smoke it!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Were tested Dornier method. Main problem is unstability final superfine material. Good energetic properties has this EM only during a few hours.
Maximal 24 hours. Superfine aluminium (or magnesium or magnalium or stone from lighter) is instantly after prepare (during prepare) in oxidizing
process on air. And any oxidizer (KClO3/4 ) in mixture, this process accelerate. Between two flat metal is not problem prepare super fine metal
powder. Problem is very low stability. Is it an the blind way..... ....LL ....LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Have you ever tried to add few gr of charcoal to prevent oxidation ? This is how dark german works
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
The process very simple yet effective. Here's a gif from the original video:

I don't see how charcoal would stop oxidation from air. It's not possible to cover the metal particles with equally sized charcoal particles and it
certainly doesn't act as a sacrificial anode.
My experience is that even submicron magnesium survives a day or so in dry air, even mixed with an oxidizer, before it really starts to lose
performance.
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I have tried Dornier's method, very ingenious, it works well. But it wont work with mild steel, one needs stainless or hardened steel. Be careful
not to over grind to.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Sorry, gif is a bit low resolution.
Are you using two smooth pieces of steel, or is the upper piece a mill file?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
The method of using a metal plate and bar is a great idea and I think the oxidation process might be able to be mitigated by using a waxy substance
such as magnesium stearate or maybe paraffin, anything that can coat the surface a slight bit and I think the grinding motion of this process is ideal
for making an extremely thin and complete coating on the surface. Maybe even some soap would work well for this, something as simple as Ivory.?
Also, would a round bar/rod work maybe better than a flat bar as it would allow for more pressure at any given point over the metal being ground.?
[Edited on 5-8-2018 by RogueRose]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
https://www.youtube.com/watch?v=ctJDPjnZ3Ag
Curious how easily one of these could be sanded down using a drill for example, compared to solid aluminium. 
[Edited on 11-5-2018 by nitro-genes]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Do any have a source for information on HNDPAN, Bis(2,4,6-trinitrophenyl)aminoethyl nitrate? Particularly looking for VOD, toxicity and storage
stability.
The information included with Axt's OTC Pentryl publication looked interesting, the Lead block expansion in particular.
Not much to be found with Google-
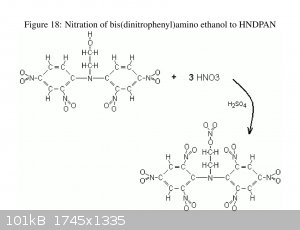
[Edited on 5-17-2018 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Plasticized nitrourea
Nitrourea decomposes in the presence of water and in contact with an alkali. What if just after it was filtered from the sulfuric acid we plasticized
it? Wouldn't it make it water-proof?
Oh, hello!  |
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What VoD NU got and how easily can be detonated ? Can it be packed at high density ? I did not found much info for NU.
[Edited on 19-5-2018 by underground]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by underground  | What VoD NU got and how easily can be detonated ? Can it be packed at high density ? I did not found much info for NU.
[Edited on 19-5-2018 by underground] |
Crystal density of 1.6 g/cm³ (1).
VoD of 6860 m/s at 1.45 g/cm³ (2)
1. http://www.chemspider.com/Chemical-Structure.56160.html
2. https://en.wikipedia.org/wiki/TNT_equivalent#Relative_effect...
Oh, hello!  |
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
interesting, i will try some once i got some free time. What i got into my mind is to keep it stored into a desiccator bag. When you want to use it,
put it into a zip bag with some liquid HE to increase the density. Or press it into high density into a plastic container and cover it with some wax.
I dont think so you could make a plastic from it being stable without storing it somehere air tide.
On the other hand NQ is almost the same as NU and it doesnt have the hydrolysis problem, but unfortunately NQ is very difficult to detonate and it is
hard to achieve high densities. My fear it that NU also will be difficult to detonate like NQ
[Edited on 20-5-2018 by underground]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by underground  |
interesting, i will try some once i got some free time. What i got into my mind is to keep it stored into a desiccator bag. When you want to use it,
put it into a zip bag with some liquid HE to increase the density. Or press it into high density into a plastic container and cover it with some wax.
I dont think so you could make a plastic from it being stable without storing it somehere air tide.
On the other hand NQ is almost the same as NU and it doesnt have the hydrolysis problem, but unfortunately NQ is very difficult to detonate and it is
hard to achieve high densities. My fear it that NU also will be difficult to detonate like NQ
[Edited on 20-5-2018 by underground] |
The binder binds the solid to the plasticizer (in this case, methylricinoleate). The oil is hydrophobic and will repel water. Assuming that your
Nitrourea is anhydrous by the time of the plasticizing process and your solvent is also anhydrous, your should obtain a putty that is 99.9% water free
and will repel it. Does this sound right?
I have never handled Nitrourea, nice to know that it is that insensitive.
Maybe using a 5-10g ETN booster would help Nitrourea go high order.
The appeal for Nitrourea is that it is easily synthesized. Urea can be bought OTC, and then nitrated with nitrating salts and dehydrated to yield
Nitrourea. I suppose that recrystallization is a must since the fertilizer might contain lots of impurities.
Urea runs for 4 usd/kg here, its pretty cheap.
http://energetics.chm.uri.edu/system/files/PEPUNNUManuscript...
This paper reports a 1.73 g/cm³ density for Nitrourea. With 10% inerts I assume it would result in a 1.55ish density.
Oh, hello!  |
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
That is true, i see your point, now i am not sure if the plasticizer will help. Why dont you try to plasticize ammonium nitrate and let is ouside. If
it absorb water then the NU will do the same hydrolizing it.
[Edited on 21-5-2018 by underground]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by underground  | That is true, i see your point, now i am not sure if the plasticizer will help. Why dont you try to plasticize ammonium nitrate and let is ouside. If
it absorb water then the NU will do the same hydrolizing it.
[Edited on 21-5-2018 by underground] |
Hm.. I thought that binding it to an oil would make it hydrophobic.
Maybe I'm wrong, I don't know.
I plasticized some dough yesterday and when a drop of water hits it, it does not mix with the putty. It simply slides through the surface and doesn't
spread at all.
Oh, hello!  |
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by joseph6355  | Quote: Originally posted by underground  | That is true, i see your point, now i am not sure if the plasticizer will help. Why dont you try to plasticize ammonium nitrate and let is ouside. If
it absorb water then the NU will do the same hydrolizing it.
[Edited on 21-5-2018 by underground] |
Hm.. I thought that binding it to an oil would make it hydrophobic.
Maybe I'm wrong, I don't know.
I plasticized some dough yesterday and when a drop of water hits it, it does not mix with the putty. It simply slides through the surface and doesn't
spread at all. |
Try it with some Ammonium nitrate and let us know
[Edited on 21-5-2018 by underground]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by underground  | Quote: Originally posted by joseph6355  | Quote: Originally posted by underground  | That is true, i see your point, now i am not sure if the plasticizer will help. Why dont you try to plasticize ammonium nitrate and let is ouside. If
it absorb water then the NU will do the same hydrolizing it.
[Edited on 21-5-2018 by underground] |
Hm.. I thought that binding it to an oil would make it hydrophobic.
Maybe I'm wrong, I don't know.
I plasticized some dough yesterday and when a drop of water hits it, it does not mix with the putty. It simply slides through the surface and doesn't
spread at all. |
Try it with some Ammonium nitrate and let us know
[Edited on 21-5-2018 by underground] |
I might, actually. I think I'll use calcium chloride instead.
Oh, hello!  |
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Question: Is there a way to estimate the VoD of an explosive at different densities giving the VoD at theoretical density?
Question number 2: Does Ammonium picrate react with metals the same way as picric acid does?
Oh, hello!  |
|
|
| Pages:
1
..
62
63
64
65
66
..
78 |