| Pages:
1
..
64
65
66
67
68
..
78 |
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That's just the analysis; it's not a good source of phosphorus pentoxide or elemental calcium. I don't think phosphorus pentoxide is available OTC. It
is available to amateurs but can be a little hard to find at times.
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by JJay  | | That's just the analysis; it's not a good source of phosphorus pentoxide or elemental calcium. I don't think phosphorus pentoxide is available OTC. It
is available to amateurs but can be a little hard to find at times. |
That sucks. :\
Where can I find elemental phosphorus OTC then? Even a child could make P2O5, you just have to burn it with enough oxygen in a dry environment.
Oh, hello!  |
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Which is the easiest way to make acetyline gas? I can not find anywhere calcium carbide and acetyline cylinders are huge and expensive. Is there any
other way to make acetyline ?
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Just type in google "synthesis of acetylene" found it on first found website
F is a wide-necked 250 ml bolt-head flask, to which is fitted a double-surface reflux water-condenser C and the dropping funnel D. From the top of C,
a delivery-tube leads down to the pneumatic trough T, where the gas can be collected in jars in the usual way. 100 ml of rectified ethanol and 25 g of
powdered potassium hydroxide are placed in the flask F. The mixture is gently boiled under reflux until the potassium hydroxide is almost entirely
dissolved. 15 ml (or 33 g) of 1,2-dibromoethane is place in the dropping funnel D, and added drop-wise into the boiling solution of potassium
hydroxide in ethanol. A rapid reaction occurs, acetylene being generated and potassium bromide precipitated. Generated acetylene is passes through the
apparatus until all the air is expelled. Then acetylene is collected under water or directly used for an experiment.
You can try this with 1,2 dichloroethane instead, which is cheaper and can be made by esterification of ethylene glycol with hydrochloric acid and see
if it works.
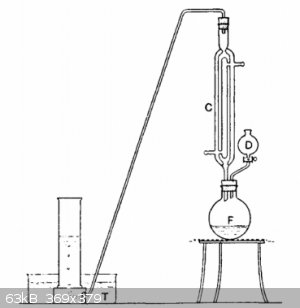
[Edited on 14-8-2018 by mackolol]
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
My purpose is only for making silver acetylide. I guess air is not a broblem as it will not react with the silver nitrate solution. So i am thinking
to pass directly the gas into the solution and skip the reflux condenser cause i dont have it
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Q. After running a solution of ETN/methanol through a filter during recrystallization i am left with an unknown white precipitate in the filter. Not
much at all, but it certainly is not ETN as i have tried to burn it but it is completely inert. It does not even melt under a blowtorch. It has the
consistency of a very fine powder and is not crystalline. Any ideas on what is it? I used the hno3/sulfuric acid route for the synthesis.
[Edited on 24-8-2018 by DrManhattan]
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DrManhattan  | Q. After running a solution of ETN/methanol through a filter during recrystallization i am left with an unknown white precipitate in the filter. Not
much at all, but it certainly is not ETN as i have tried to burn it but it is completely inert. It does not even melt under a blowtorch. It has the
consistency of a very fine powder and is not crystalline. Any ideas on what is it? I used the hno3/sulfuric acid route for the synthesis.
[Edited on 24-8-2018 by DrManhattan] |
No idea... Maybe it's some kind of impurity in your sulfuric acid?
I have seen weird things with AN/H2SO4 nitrations of impure erythritol (namely Truvia). I often had a bit of precipitate that was insoluble in water,
alcohol or acetone. Maybe it's the same compound, I never bothered to do anything but dispose of it.
After going to pure erythritol and HNO3 rather than raw Truvia and nitrate salt I've never had this happen.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Magnesium nitrate as a dehydrating agent
When magnesium nitrate forms it tends to absorb water forming a hexahydrade. What about using it in the rdx preparation ? In theory if you add
mangesium metal into nitric acid it will concentrate it. Has anybody ever tried it ?
[Edited on 17-9-2018 by underground]
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Hi, just wondering if Mercury Fulminate is compatible with aluminium as i have heard most people say that brass should be used for a mercury fulminate
cap.
|
|
|
walruslover69
Hazard to Others
  
Posts: 216
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
I would not recommend using anything aluminium with mercury or its salts.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Mercury fulminate reacts rapidly with aluminium forming Al2O3 when in direct contact. The trace amounts of elemental mercury within the product would
as well forming amalgam. Copper is another no no.
I have read that it reacts much slower with other metals like brass and bronze but faster if moisture is present. One metal that ot does not react
with is nickel though.
You can either use a more compatible metal and apply a protective coating to the surface so it does not come in direct contact or use another primary.
Hope that helps.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Exactly the answer i was looking for. Many thanks.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Copper has been used for MF during WW1 and I've seen a good deal of copper in WW2 items aswell.
Are you certain about this ?
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I will check some literature when I get home but I am quite sure that the copper was coated in a protective lacquer. I will get back to you
though
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
From Primary explosives, pg 48;
"Moist MF reacts with copper giving copper fulminate which is less sensitive to impact but more sensitive to friction the MF itself. The presence of
moisture is necessary for the formation of copper fulminate. Another side effect the reaction of copper is the precipitation of mercury which forms
an amalgam that may weaken the copper cap which the MF is embedded in.
It is therefore necessary to prevent contact of MF with copper in its applications protective coating or nicking of the copper surface."
Seems like these reactions are very slow though but my money would be on the fact that these WWll copper detonators would have been protected in some
way from direct contact with the mercury fulminate.
[Edited on 12-11-2018 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
An idea that i was thinking for a long time but i did never posted cause i thought that maybe it will be a dump idea but i decided to post it anyway
:p
I was thinking if it is possible to increase the density of an energetic material by cooling it.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Cooling the energetic material could indeed improve it's density, though how much would really depend on phase transition temperatures and on the
chemical structure. Water for example forms an extensive hydrogen bond network on cooling, making ice actually less dense than water itself. For most
compounds shrinkage on cooling is only a few percent, so the effect for most compounds would probably not be very significant. It is an interesting
question though what would happen with the detonation velocity at very low temperatures, it is well known that some primaries only make DDT reliably
at warmer temperatures and can fail when cold (DDNP for example). If anything, I would say preheating a explosive could potentially increase its
detonation velocity (Not sure though).
Hmmm, has this ever been tested? Take the most dense and heat stable explosive, heat it to the brim of its existence, detonate and measure VoD
compared to cold? 
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Cooling a HE would indeed increase its detonation velocity and detonation pressure. Those properties are much more strongly affected by density than
energy of the material, where the thermal energy is included. Initiating detonation would probably be more difficult as you say nitro-genes, since the
compound needs more of a kick to start a self sustaining detonation. I remember reading something about hot molten TNT being almost as sensitive as NG
but it surely has lower VoD. I also think Urbanski listed the VoD of frozen NG to 8000 m/s, quite a bit higher than the normally reported 7700 m/s.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yea that is the point. For example maybe cooled PETN or ETN could achieve density and peformance like HMX or even higher.
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Bought a bunch of "Calcium Ammonium Nitrate" cold packs today from the store. However these things are rarely what they said so I tested it with
Copper and some Sulfuric Acid, expecting a precipitate of Calcium Sulfate and emission of brown gas. The gas was produced but no precipitate occurred.
Is Calcium Sulfate soluble in highly acidic solutions or is my salt likely just Ammonium Nitrate? Its hydrated but not hygroscopic.
|
|
|
walruslover69
Hazard to Others
  
Posts: 216
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
try to get a precipitate with sodium sulfate, or sodium carbonate
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Is Lead Styphnate the "best" primary explosive ?
Edit^
I'm sure its not. I'm just asking because it is used in almost all commercial explosives
[Edited on 13-1-2019 by feedme94]
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
No, its used as the first kickstart in a blastingcap, because ist very flame sensitive. The leadazide that follows is the real initiating compound. So
beter for dextrinated leadazide, since it also quite sensitive towards other, safer priming compoundss. For example blackpowder.
Furthermore, there are better primarys (tetrazoles, which also have drawbacks btw). Go for BNCP is you want the holy grale in my opinion.
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
At the moment im on my way for SADS and TACP
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I want to work with hydrazine hidrate and I want to ask about protective equipment required .I'll work out and wear gloves but I need to use the gas
mask?
|
|
|
| Pages:
1
..
64
65
66
67
68
..
78 |