deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Speculative reaction of alcohols to carbonic acids with Mg and CO2
| Quote: | | @deltaH: magnesium reacts with alcohols to give the alcoxide, i.e., it reduces the hydroxyl hydrogen rather than the ipso-carbon.
|
Thanks, that this is what I thought too last night, but then I followed through on a possible surface mechanism in the pressence of CO2,
bear with me:
(i) iPrOH + e- => iPrO- + H*
(ii) iPrO- + CO2 => iPrOCO2-
(iii) iPrOCO2- + e- => iPr* + CO32-
See what I mean?
[Edited on 18-11-2013 by deltaH]
[Edited on 18-11-2013 by vulture]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
UC:
Isopropylchloride is added, reaction starts, vigorous boiling ensues, iPrCl evaporates, reaction is starved of iPrCl and slows down. iPrCl condenses
back into reaction mixture, reaction starts again, etc...
deltaH:
The formation of a hydrogen radical is extremely disfavoured.
However, a quick google search (!) revealed the following: http://digital.library.okstate.edu/oas/oas_pdf/v23/p67_68.pd...
If this is correct, iPrOH + NaOH + CO2 would be the best way to go, no need to waste Mg.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by vulture  | UC:
Isopropylchloride is added, reaction starts, vigorous boiling ensues, iPrCl evaporates, reaction is starved of iPrCl and slows down. iPrCl condenses
back into reaction mixture, reaction starts again, etc...
deltaH:
The formation of a hydrogen radical is extremely disfavoured.
However, a quick google search (!) revealed the following: http://digital.library.okstate.edu/oas/oas_pdf/v23/p67_68.pd...
If this is correct, iPrOH + NaOH + CO2 would be the best way to go, no need to waste Mg. |
H* star isn't a hydrogen radical, I'm from a heterogeneous catalysis background, we write * species to mean surface species (sorry for the confusion).
These surface hydrogens are not very energetic, it's not nascent hydrogen or some such nonsense! I was considering them to be 'surface hydrides' of
sorts. It is not unreasonable to suspect the surface Mg-H would quickly react with iPrOH (acting as a weak acid) to form Mg-OiPr + H2(g). So these
hydrides will be quickly mopped up under the conditions.
As the grignard reagent mechanism proceeds as a surface reaction, I simply traced it through to suggest that it may not be as 'impossible' as one
might think and may warrant investigation.
In regards to your reference, this stops neatly at a simple organic carbonate formation (after all, you have only reacted it with a base, not a
metal), however, in what I'm proposing, you have formal single electron reduction steps (on the carbonate intermediate as well) and could generate the
surface bound iPr* specie by such a mechanism, from there, the reaction could proceeds with surface CO2 to form iPrCO2- and finally desorption to form
Mg(iPrCO2)2.
With NaOH, you will simply and at best form isopropyl carbonates, you need the reducing agent to do otherwise via the mechanism I am suggesting.
The complete mechanism then would be (note: *=chemisorbed surface bound species, not radicals but organometallically bound to magnesium metal or
simply surface 'salts', think of it as Mg-X, also note, I've modified the steps to be in surface notation consistently to avoid confusion and ditched
the formal charge notation mixed with charges, it's just a little simpler this way. The reduction steps are the ones where the *'s don't ballance,
i.e. step (i) and (iii)
(i) iPrOH(l) => iPrO* + H*
(ii) H* + iPrOH(l) => iPrO* + H2(g)
(ii) iPrO* + CO2 => iPrOCO2*
(iii) iPrOCO2* + * => iPr* + CO3*
(iv) iPr* + CO2(g) => iPrCO2*
Also cleaned up some typos from before, though there may still be some left.
or as charged species, just remember that they are surface species:
(i) iPrOH(l) + 2e-=> iPrO- + H-
(ii) H- + iPrOH(l) => iPrO- + H2(g)
(ii) iPrO- + CO2 => iPrOCO2-
(iii) iPrOCO2- + 2e- => iPr- + CO32-
(iv) iPr- + CO2(g) => iPrCO2-
Note, I was incorrect to call these single electron reductions previously, in full charge notation, they clearly are double electron reduction steps!
Again, this is a proposed mechanism, not necessarily 'truth'!
[Edited on 18-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Ah crap, I got confused again. 
Anyway, this shows that reactions with alkoxides results in carbonates, ergo, the reaction you propose cannot work.
| Quote: |
It is not unreasonable to suspect the surface Mg-H would quickly react with iPrOH (acting as a weak acid) to form Mg-OiPr + H2(g). So these hydrides
will be quickly mopped up under the conditions.
|
Where does the hydride come from? You have just magnesium metal. This could react with iPrOH to form hydrogen, but this doesn't involve any hydrides
(which are by definition, species of H-).
You are then left with the magnesium alkoxide, which cannot form the acid in any conceivable way.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | Where does the hydride come from? You have just magnesium metal. This could react with iPrOH to form hydrogen, but this doesn't involve any hydrides
(which are by definition, species of H-). |
These are elementary steps, you only have one hydrogen from
iPrOH, so you can't form H2 in one step, you first need to dissociate iPrOH, that combined with reduction generates surface hydride and iPrO- as I've
written, then the surface hydride can react with another iPrOH to form H2(g). I was just writing my equations as a series of elementary reaction steps
(I hope).
As for your second point, as per my mechanism, the conceivable way is that the alkoxide forms an alkyl carbonate on the surface and this is then
reduced and dissociated into carbonate and a surface alkyl magnesium specie (I've written it as iPr- in charge notation, but it's technically a
surface iPr-Mg animal). This then goes on to react with CO2 to form iPrCO2-.
I don't see how this is very unreasonable. One might argue that the surface get's 'poisoned'/passivated by surface carbonates, but then again, you're
also generating acid, so potentially dynamically this still works, it simply runs slower because of the surface carbonate. In many heterogenous
catalyst kinetics involving CO2, even when CO2 is a reagent (e.g methanol synthesis from CO/CO2/H2), one finds CO2 terms in the denominator as well
because of the inhibition due to occupation of surface sites. Not that I'm suggesting magnesium is catalytic here, it is formally a surface reagent
where Mg is a reagent and consumed, however, surface reactions and heterogeneous catalysis mechanistically are similar things, the only difference is
that overall in catalytic mechanisms, the surface sites are regenerated and catalyst is not consumed overall, of course.
This might be a similar issue here, but again, this doesn't necessarily mean it can't work, it's just a question of if one can't get it to work, that
might be the reason 
I would 'start' this reaction with Mg and iPrOH (maybe with a couple of I2 crystals) only and once hydrogen production is visible, throw in the dry
ice. Of course, everything is going to slow to a crawl (possibly completely stop) then, but perhaps if one leaves this in an open dewar for several
hours with a LOOSE venting lid, it might just go with enough time. It's easy enough to try for some.
Failing that, one would have no choice to carry this out at higher temperatures using CO2 gas if there are serious kinetic issues (which I suspect
there would be).
Hey, I never said it's gonna be easy 
[Edited on 18-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
As for your second point, as per my mechanism, the conceivable way is that the alkoxide forms an alkyl carbonate on the surface and this is then
reduced and dissociated into carbonate and a surface alkyl magnesium specie (I've written it as iPr- in charge notation, but it's technically a
surface iPr-Mg animal). This then goes on to react with CO2 to form iPrCO2-.
|
I don't see how you would go from isopropyl carbonate to iPr-. Carbonates are very stable animals and very resistant to reduction, that's why they can
be used as solvents for alkali metal production for example.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I hear you, but alkyl carbonates are also alkylating agents... granted weak ones, but nevertheless. You must have heard of the use of dimethyl
carbonate as a 'green' methylating reagent with strong basis like DBU as catalysts. So the R-OCO2- bond is cleavable catalytically. In this
role the surface of the magnesium behaves a bit like a ordinary metal catalyst, it may help the bond dissociation, but also as a strong base. Why?
Because a surface bound R* and CO3* that would form in the mechanism are probably energetically quite stable, well the CO3* hugely so. Also, the
magnesium metal, being so electropositive, is also a strong base. For example, a lewis base wants to donate a lone pair of electrons, in this case,
the magnesium wants to push electrons off too and so form the acidic free carbonate and so ultimately the salt.
When you think about it that way, it's maybe not so hard to envision alkyl carbonate intermediates on the surface of magnesium being readily cleaved.
Thermodynamically, making MgCO3 is a very steep downhill!
My only big worry here is the retardation/surface passivation by CO2. This is particularly a concern if you chuck in dry ice, because the solution
will cool down all the way because isopropanol only freezes at -89C. The result is you will probably dissolve a huge amount of CO2 in your solution
and poison/passivate you magnesium surface by swamping it? This is a great shame if this is the case for it would be trivially easy for the amateur
chemist to carry this out with dry ice.
Maybe the thing to do is to stick a thermometer into the solution, and add dry ice a little at a time to keep the temperature between a range, say
0-10C.
Then there's the question of the effect of moisture, would moisture be a killer here like it is for grignards? My gut feeling says not, because water
will simply form MgO on the surface and that would quickly form MgCO3, so as long as the MgCO3 is not stopping the reaction, the MgO won't either (it
would just waste a tiny amount of Mg).
In the end of the day, it will be VERY easy to see if this process cannot proceed because of passivation by CO2, it will simple stop running if this
is the case and you can see this as gas bubble will stop being evolved from the magnesium turnings.
Sorry for waffling, I have so much to say on this, but maybe for another time...
[Edited on 18-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I hear you, but alkyl carbonates are also alkylating agents... granted weak ones, but nevertheless. You must have heard of the use of dimethyl
carbonate as a 'green' methylating reagent with strong basis like DBU as catalysts.
|
I've used ethylene carbonate for alkylation before, so yes, it does work. However, magnesium is not a nucleophile in the classical sense and I doubt
very much it will attack carbonates. The attack of magnesium on alkylhalides does not proceed through a two electron mechanism, see the attached PDF.
If anything happens, I'd say that the isopropoxide attacks the carbonate forming diisopropylether and magnesium carbonate. This is a far lower energy
pathway too.
[Edited on 18-11-2013 by vulture]
Attachment: Nieuw document 3.pdf (266kB)
This file has been downloaded 473 times
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | I've used ethylene carbonate for alkylation before, so yes, it does work. However, magnesium is not a nucleophile in the classical sense and I doubt
very much it will attack carbonates. |
Having magnesium dissociate an alkyl carbonate is probably not likely, but that's going from the wrong direction. I think you are right, if a dialkyl
carbonate forms, then this would desorb as a byproduct and little chance of it being dissociated again on kinetic arguments. Let me elaborate:
The forward reaction: iPrOCO2- + e- => iPr* + CO32-, makes CO32- which means this
step is probably very favoured thermodynamically.
However, for magnesium to break up a carbonate, the first step would have to be something like: R-OCO2-R => R-O2C-* + R-O-*, this
doesn't look favoured because neither the metal ester species (or whatever you would call this) R-O2C-* nor the alkoxide R-O-* are particularly low
energy products.
However, from the other direction you get to make carbonate in the step which is very favoured.
[Edited on 18-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
The forward reaction: iPrOCO2- + e- => iPr* + CO32-, makes CO32- which means this step is probably very favoured thermodynamically.
|
Nope, iPr* is a very high energy fragment and breaking a C-O bond is endergonic. Furthermore, the elimination of CO32- is also
disfavored because (a) you're not creating any new bonds, (b) you are creating a moiety with a higher charge density and (c) the product is not
removed from the reaction mixture (as would be the case for CO2 in case of alkylation with dicarbonates).
| Quote: |
However, for magnesium to break up a carbonate, the first step would have to be something like: R-OCO2-R => R-O2C-* + R-O-*, this doesn't look
favoured because neither the metal ester species (or whatever you would call this) R-O2C-* nor the alkoxide R-O-* are particularly low energy
products.
|
The diester is not present in this reaction. Magnesium does not play a role in this except maybe forming the alkoxide from the alcohol. I do not
understand why you wish to force one electron reductions by magnesium into this.
Furthermore, alkoxides are perfectly stable and much lower energy than carbon radicals.
I split the thread because this discussion has nothing to do anymore with grignard reagents.
[Edited on 18-11-2013 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks for splitting this off, shame we were really cluttering up that thread, sorry, but this topic is so interesting!
iPr* is not a radical, its a surface species and the carbon is formally bonded to magnesium, it is organometallic in nature, probably closest to
iPr-Mg-, here I'll draw it:
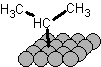
This is the same kind of bond (half of it anyway) as what forms in a grignard reagent, except that it's lower in energy because the magnesium is not a
single Mg atom, but part of a surface.
As for breaking a C-O bond, this certainly is endothermic, but you're making a magnesium carbonate from the break and that is thermodynamically very
favoured.
| Quote: | | Furthermore, the elimination of CO32- is also disfavored because (a) you're not creating any new bonds, |
You're making magnesium carbonate, an ionic salt, forget about the CO2 fragment in this for now, this step energetically boils down
to going from magnesium to a magnesium oxide (the CO2 simply neutralises the oxide but that doesn't affect the dH of this step that much).
You should try to see things more broadly, not just in terms of simple bond breaking and formation. In this case you break a covalent C-O bond and
make a ionic Mg2+ O2- effectively or in words, you reduce a carbon oxide bond and oxidise magnesium metal to form a magnesium oxide.
| Quote: | | (b) you are creating a moiety with a higher charge density |
It's a very stable ionic salt, I don't see how
this is bad thing in this case. I've often see organic chemists use arguments like this, while it might be applicable to certain cases (particularly
in solution), it fails with solids and the like, here you simply look at dH of steps.
| Quote: | | c) the product is not removed from the reaction mixture (as would be the case for CO2 in case of alkylation with dicarbonates).
|
Yes it is because of the very low solubility of magnesium carbonate. It is far less soluble in fact than
what CO2 is in this system. This also helps you.
As for the rest, again not radicals.
*************
Thanks for your lively input vulture, it's interesting. You've also brought to light many alternatives and other reactions that may dominate. It's
been interesting exploring the concept with you.
I think I will try this reaction someday as it looks really fascinating and not too difficult to carry out. It would be nice to see what exactly ends
up forming in the presence of CO2 
[Edited on 18-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Your iPr-Mg species, or whatever you want to call it, cannot exist.
To form it from Mg(OiPr)2 while producing CO32-, you would end up with a (MgiPr)+ moiety. Part of a
surface or not, this is not stable.
If you write down the reaction in full it's easy to see:
Mg(OiPr)2 + 2 CO2 --> Mg(OCO2iPr)2 This is already highly speculative.
But now the madness really begins:
Mg(OCO2iPr)2 + 2Mg --> 2(MgiPr)+ + MgCO3 + CO32-
| Quote: |
but you're making a magnesium carbonate from the break and that is thermodynamically very favoured.
|
Look at the structure of the isopropylcarbonate magnesium salt, the carbonate salt is already there, you're only splitting off the isopropyl. (Drawing
the structure will make it obvious). Furthermore, the reaction pathway I proposed, whilst also highly speculative, involves the formation of magnesium
carbonate plus a stable organic molecule.
| Quote: |
this step energetically boils down to going from magnesium to a magnesium oxide
|
No it does not. Are the formation enthalpies of magnesium oxide and magnesium carbonate the same? No.
[Edited on 18-11-2013 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | To form it from Mg(OiPr)2 while producing CO32-, you would end up with a (MgiPr)+ moiety. Part of a surface or not, this is not stable.
|
I don't agree that you can talk about a "(MgiPr)+ moiety" for a bulk metal surface, there are no discrete charges here per say like that, only in
solutions. If you mean to say a chemisorbed surface alkyl species is not stable, that is not true... most heterogeneous catalysis relies heavily on
the stability of organometallic surface alkyl groups... the classic case being Fischer-Tropsch where you have a growing chain of hydrocarbon of the
type: M-CH2-CH2-.... These are so stable that you have to heat them over 300C to get them to desorb (break off) as the olefin. In Fischer-Tropsch
reactions, iron or cobalt are the typical metal catalysts used. Magnesium simply changes the character of surface bonds to be more polarized because
it is so electropositive a metal.
| Quote: | | Mg(OiPr)2 + 2 CO2 --> Mg(OCO2iPr)2 This is already highly speculative. |
Everything here is speculative,
but if you mean highly unlikely, I also have to disagree, because this is simply a carbonate formation step with a nucleophilic group (alkoxide)
attacking carbon dioxide. It's no stranger than OH- + CO2 => HOCO2-. In fact this step is probably the least doubtful step in the whole mechanism.
That is why I dislike this charge notation because it creates a hell of a lot of confusion. Chemists want to impose these because it is what they are
used to in solution chemistry, but this simply does not apply for surfaces. Bulk metals and periodic solid systems of this type do not have discrete
fixed orbital occupancy, they have a band structures. The surface is peppered by all kinds of surface bound groups: carbonates, alkyls, alkoxides,
carbon dioxide. Importantly, the whole thing is charge neutral. To speak of formal charges is almost meaningless for these systems because of the
delocalisation of electrons into bands over the whole system. You can only say things like, the bond is very polarised (which is true for O-Mg and
C-Mg bonds).
Even a surface alkoxide is not a R-O-, it is R-O-M where the -O-M bond on the surface is highly polarised, but not 1+ on the magnesium and
1- on the oxygen. Sometimes these surface groups don't even sit end-on (bound to one surface atom), but sit in between two even three surface metal
atoms, depending on what is energetically favoured for it (though I suspect for magnesium the end on is most stable).
| Quote: | | No it does not. Are the formation enthalpies of magnesium oxide and magnesium carbonate the same? No. |
You
have misunderstood what I was trying to say, I said take out the CO2 contribution because you are not forming carbon dioxide from carbon and oxygen
(or carbonate from carbon and oxygen), but you are oxidising magnesium a fresh and this is the main contribution to the dH of that step, let me write
it out:
Mg(s) + CO32- => MgCO3(s) + 2e-
On both sides you have carbonate, so that is not what is making this reaction so exothermic, what makes it so exothermic is the oxidation of
magnesium. That is what I was trying to say. You are right the bonds on the carbonate doesn't change much, but that is not the highly exothermic step
here, it is the magnesium oxidizing that drives this thing.
This is why I compared it to magnesium oxidizing to magnesium oxide which has very large negative dH.
[Edited on 19-11-2013 by deltaH]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I don't agree that you can talk about a "(MgiPr)+ moiety" for a bulk metal surface, there are no discrete charges here per say like that, only in
solutions. If you mean to say a chemisorbed surface alkyl species is not stable, that is not true... most heterogeneous catalysis relies heavily on
the stability of organometallic surface alkyl groups... the classic case being Fischer-Tropsch where you have a growing chain of hydrocarbon of the
type: M-CH2-CH2-....
|
I'm sorry but you are cherrypicking and comparing apples to oranges.
Fischer-Trops is (a) catalytic and (b) involves transition metals. In this reaction, (a) magnesium is not catalytic and (b) not a transition metal
(duh). Which you hint at yourself:
| Quote: |
Magnesium simply changes the character of surface bonds to be more polarized because it is so electropositive a metal.
|
The reaction you propose clearly does not fall in the category of heterogenous catalysis. If you keep dragging in unrelated subjects on the basis of
false association, there is no point in discussing with you.
| Quote: |
That is why I dislike this charge notation because it creates a hell of a lot of confusion. Chemists want to impose these because it is what they are
used to in solution chemistry, but this simply does not apply for surfaces.
|
Yes, chemists are in a conspiracy to discredit heterogenous catalysis and surface chemistry. Obviously.
| Quote: |
Importantly, the whole thing is charge neutral. To speak of formal charges is almost meaningless for these systems because of the delocalisation of
electrons into bands over the whole system. You can only say things like, the bond is very polarised (which is true for O-Mg and C-Mg bonds).
|
Take a step back and think about what you just wrote. This proposed reaction does involve highly polarized bonds. We have hard
nucleophiles and electrophiles here.
The metal may be charge neutral in total, but that does not relieve you from conservation of charge of the
whole reaction systemin general. You are still missing an electron in your case.
| Quote: |
You have misunderstood what I was trying to say, I said take out the CO2 contribution because you are not forming carbon dioxide from carbon and
oxygen (or carbonate from carbon and oxygen), but you are oxidising magnesium a fresh and this is the main contribution to the dH of that step, let me
write it out:
Mg(s) + CO32- => MgCO3(s) + 2e-
On both sides you have carbonate, so that is not what is making this reaction so exothermic, what makes it so exothermic is the oxidation of
magnesium. That is what I was trying to say. You are right the bonds on the carbonate doesn't change much, but that is not the highly exothermic step
here, it is the magnesium oxidizing that drives this thing.
|
You might want to write it as Mg --> Mg2+ + 2e-...
You are conventiently forgetting the formation enthalpy of your iPrMg species. Which is probably off the charts.
| Quote: |
This is why I compared it to magnesium oxidizing to magnesium oxide which has very large negative dH.
|
This is the whole problem with your approach. You are using a false comparison to support your claim, while ignoring basic chemistry (stability of
radical or highly charged species) that discredits your hypothesis.
[Edited on 19-11-2013 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I didn't mean to imply that this is about catalysis per say, it's about surface chemistry, which is applicable for heterogeneous catalysts or metal
fluid reactions. The difference here is that your metal is consumed overall by formation (ultimately) of salts which can leach off because of the
presence of solvent. This hopefully forms growing precipitate sludges and do not simply passivate and stop at the metal surface.
We have a difference of opinion on some steps, the formation of iPrOCO2- and it's subsequent reductive dissociation to form surface metal
alkylates (what you call radicals) and carbonates. I think it's reasonable, you think it's not, we've both made our arguments in support of our
thinking.
By the way, while I have had my disagreements with you in the past, I want to commend you on holding a scientific argument/debate, without resorting
to insults and such. While I disagree with you, you have done a sterling job of debating in a civil fashion. I think this sets a fantastic example for
all of us to follow.
Cheers!
[Edited on 19-11-2013 by deltaH]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The problem is that anyone can write out a reaction scheme that is balanced and looks very nice on paper. But the key is that the direction could go
either left to right or right to left. If the reaction of the "products" back towards the "reactants" releases energy (exothermic) and is favored
entropically, then the reaction will not go forward as written. Also, a catalyst can only change the rate of a reaction, not make one go in the
opposite direction of what nature intended. So it does not matter what catalyst or solid surface you have, some reactions will not for forward, with
or without a catalyst, (unless you are adding energy to the system). So whether you are talking bulk chemistry, surface chemistry, solution, solid,
heterogeneous, homogenous catalyst, etc, if the reaction is unfavorable, the reaction has to release energy (as written) to move forward, unless you
are driving it some other way.
EG,
2 H2O ---> H2(g) + O2(g) does not spontaneously happen from left to right, as the opposite reaction (right to left) is quite exothermic.
No catalyst will change that, but applying an electrical potential to the reaction can shift the direction, by applying energy to shift the
equilibrium of the reaction.
|
|
|
Nicodem
|
Thread Moved
19-11-2013 at 07:23 |
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
"There is no need to argue if an experiment can be made." - H. St. C. Deville
|
|
|
|