| Pages:
1
2
3
4
..
6 |
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
By the way, the pdf file it is said a 50:50 mixed acid, i juess it is on mls, for example it is said "65 ml of mixed acid (HNO3+H2SO4, 50:50%)" so i
guess the mixed acid it is going to be 32,5ml of NA and 32.5ml of SA, that is correct ?
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Quote: Originally posted by underground  | | By the way, the pdf file it is said a 50:50 mixed acid, i juess it is on mls, for example it is said "65 ml of mixed acid (HNO3+H2SO4, 50:50%)" so i
guess the mixed acid it is going to be 32,5ml of NA and 32.5ml of SA, that is correct ? |
yes, im pretty sure...
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Thanks!
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Negative, in such texts weight is always used, but not volume. It means 100 gr of nitric acid and 100 gr of sulfuric one.
Women are more perilous sometimes, than any hi explosive.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by caterpillar  | | Negative, in such texts weight is always used, but not volume. It means 100 gr of nitric acid and 100 gr of sulfuric one. |
100g H2SO4 x (1 mL / 1.84g) = 54.35 mL H2SO4
100g HNO3 x (1 mL / 1.51g) =66.23 mL HNO3
Total = 120.58 mL so surely it is not 100gr of NA and SA
I also believe that it is by volume.
[Edited on 31-1-2014 by underground]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This is something that caught my notice and I posted a link 9 years ago about the dinitrourea being of interest with regards to keto RDX.
http://www.sciencemadness.org/talk/viewthread.php?tid=4729&a...
The 50/50 mix of 95% H2SO4 and 100% HNO3 was by weight proportions, to clear up any question about that.
I suspect that the mixed RDX / keto RDX product may defy separation because it may be an adduct that is formed.
This process might be adaptable to use of a nitration mixture using a nitrate with H2SO4 either fully or in part. And I think adding the urea value
as urea nitrate could be useful also.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Rosco Bodine  | This is something that caught my notice and I posted a link 9 years ago about the dinitrourea being of interest with regards to keto RDX.
http://www.sciencemadness.org/talk/viewthread.php?tid=4729&a...
The 50/50 mix of 95% H2SO4 and 100% HNO3 was by weight proportions, to clear up any question about that.
I suspect that the mixed RDX / keto RDX product may defy separation because it may be an adduct that is formed.
This process might be adaptable to use of a nitration mixture using a nitrate with H2SO4 either fully or in part. And I think adding the urea value
as urea nitrate could be useful also. |
Thanks for your informations Rosco Bodine. Indeed it is by weight. I am almost sure that some nitrate salts will do the job, as long as there are
plentful out there, (kno3, nano3, nh4no3) and also i believe that K-6 is the only way for an amateur chemist for something more powerfull than RDX.
Surely when i have time i will give it a try, it looks the only way road energetic material in my opinion.
The use of urea nitrate in case of urea i also believe that it will improve the yelds of dinitrourea, and also the use of HDN in case of hexamine will
be also fine to improve the yields of K-6
Here is also an another pdf file according to k-6. I upload it here to have them all together
[Edited on 31-1-2014 by underground]
Attachment: Synthesis+of+Keto-RDX+and+its+Characterizations+Calculation.pdf (1.1MB)
This file has been downloaded 946 times
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Most likely, nitrourea would be better. But one question still remains: how strong sulfuric acid must be. Mixed acid must be strong enough to
incorporate the second nitro- group into nitrourea. I suspect, that ordinary sulfuric acid cannot do it and oleum is a must.
Women are more perilous sometimes, than any hi explosive.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Surely oleum will increase the yields of dinitrourea but it looks like it is possible just with a NA/SA mixed acid.
An 95% SA and 100% NA is described in this pdf without actually any oleum, and as you said, if you replace the urea with nitrourea it would be even better.
[Edited on 31-1-2014 by underground]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This is not a new idea. This was being discussed 10 years ago at the E&W forum and initial experiments reported that using NH4NO3 plus H2SO4
failed to produce dinitrourea. Experiments with H2SO4 plus other nitrates such as the anhydrous eutectic of 65% Mg(NO3)2 / 35% KNO3 were not
investigated.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by underground  | Surely oleum will increase the yields of dinitrourea but it looks like it is possible just with a NA/SA mixed acid.
An 95% SA and 100% NA is described in this pdf without actually any oleum, and as you said, if you replace the urea with nitrourea it would be even better.
[Edited on 31-1-2014 by underground] |
NH or Urea structure could be nitrated by Ac2O/HNO3, or HCl/Ac2O/HNO3 and some could be nitrated by HNO3/P2O5
[Edited on 31-1-2014 by DubaiAmateurRocketry]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I don't know about Keto-RDX that much, but can it be an accessible for everybody cap basecharge option for example? If one doesn't even have
eritrithol but has hexamine?
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
The point is to make something really good that it does not need exotic chemicals like Ac20 / P205
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
P2O5 is exotic? That's interesting, because I had it when I was young. No problem, a special shop with chemicals. But I think, in this particular case
anhydrous Ca(NO3)2 + H2SO4 will be better. I have a solid guess, that in such mixture CaSO4 extracts some water and increases concentration of mixed
acid. Yeah, there is one disadvantage- white mess of small crystals of CaSO4. But upper layer is transparent and can be decanted. I used it for
preparation of NG and NC.
Women are more perilous sometimes, than any hi explosive.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What is the difference between the 1,3,5-trinitro-1,3,5-triazacyclohexane-2-one and 2-Oxo-1,3,5-trinitro-1,3,5-triazacyclohexane
(keto-RDX) ? I guess it is the same. This is because in this abstract it is said that:
"Compound XIV (1,3,5-trinitro-1,3,5-triazacyclohexane-2-one), a powerful explosive, is stable under normal conditions but decomposes
to 1,3,5-trinitro-1,3,5-triazapentane in dioxane and to methylene dinitramine salts in an aqueous medium upon contact with moisture, amines,
bases, alkalis, metals, etc."
[Edited on 5-2-2014 by underground]

[Edited on 5-2-2014 by underground]
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
KCN's like KOH or NaOH. It's like lye. A little wetness (many cyanides are quite hydroscopic) and it will burn your skin like lye does. I'd think
that it's horribly corrosive tissue effect is enough to command respect. Oh yeah, and don't dump a ton of it into an acidified toilet, close the
bathroom door, turn off the fan, and sit on the throne, inhaling that remotely pleasant prussate smell - bad imitation of benzaldehyde smell.
Avoid - I said - AVOID the grape Kool-Aid at ALL costs.
"Old men who speak of victory
shed light upon their stolen life
they - drive by night- and act as if they're
moved by unheard music." B. Currie
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I can not understand exactly the procedure is described on this page about K-6 but it looks like it can be formed from nitroguanidine. So i was wondering if we can replace the urea with nitroguanidine
from traditional K-6 proccess (with 50:50 ratio mixed acid) to form K-6 is going to work, as long as nitroguanidine is much more easier to form than
nitrourea , and more stable also.
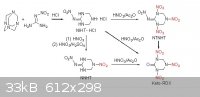
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
The NTNHT intermediate itself in the above proposed reaction looks quite energetic.
I can't name it properly ... Google yielded nothing.
Anyone know it's proper name ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Motherload  | The NTNHT intermediate itself in the above proposed reaction looks quite energetic.
I can't name it properly ... Google yielded nothing.
Anyone know it's proper name ? |
I found some info about NIHT.HCL
Go to page 71 at this file
NIHT.HCL may will be something useful for making some EM like K-6
I believe that replacing the hexamine with NIHT.HCL from RDX process with fuming nitric acid, it is going to form pure K-6
Attachment: NIHT.HCL.zip (259kB)
This file has been downloaded 621 times
[Edited on 15-3-2014 by underground]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by underground  | | What is the difference between the 1,3,5-trinitro-1,3,5-triazacyclohexane-2-one and 2-Oxo-1,3,5-trinitro-1,3,5-triazacyclohexane
(keto-RDX) ? |
None. They are just named differently.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Will urea nitrate or nitrourea is going to react with hexamine with the presence of HCL, as nitroguanidine do ? Then, what this reaction is going to
form ?
Is it possible to form something like that ?
[Edited on 17-3-2014 by underground]
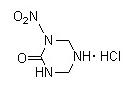
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by underground  | Will urea nitrate or nitrourea is going to react with hexamine with the presence of HCL, as nitroguanidine do ? Then, what this reaction is going to
form ?
Is it possible to form something like that ?
[Edited on 17-3-2014 by underground] |
It needs to be tested, but while nitroguanidine is relatively stable towards hydrolysis as its dervivatives NIHT.HCl; the nitrourea and its putative
derivative will be more prompt to hydrolysis... So you will probably get ring opening, decarboxylation to get O2N-NH-CH2-NH-CH2-NH2 wich should be
unstable in HCl and the final product would then be hexamine dihydrochloride and depending on the condition methylamine, dimethylamine, trimethylamine
hydrochloride and NH4Cl.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  |
It needs to be tested, but while nitroguanidine is relatively stable towards hydrolysis as its dervivatives NIHT.HCl; the nitrourea and its putative
derivative will be more prompt to hydrolysis... So you will probably get ring opening, decarboxylation to get O2N-NH-CH2-NH-CH2-NH2 wich should be
unstable in HCl and the final product would then be hexamine dihydrochloride and depending on the condition methylamine, dimethylamine, trimethylamine
hydrochloride and NH4Cl. |
So that it is why they use nitroguanidine and not nitrourea.
PHILOU Zrealone will just H2SO4/Nitrate salt (like dry AN) will yeld k-6 from nitrated NIHT.HCl or not?
Edit: Also K-6 is stable towards hydrolysis, as long as it also contains an urea group, or it is not ?
[Edited on 18-3-2014 by underground]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by underground  | Quote: Originally posted by PHILOU Zrealone  |
It needs to be tested, but while nitroguanidine is relatively stable towards hydrolysis as its dervivatives NIHT.HCl; the nitrourea and its putative
derivative will be more prompt to hydrolysis... So you will probably get ring opening, decarboxylation to get O2N-NH-CH2-NH-CH2-NH2 wich should be
unstable in HCl and the final product would then be hexamine dihydrochloride and depending on the condition methylamine, dimethylamine, trimethylamine
hydrochloride and NH4Cl. |
So that it is why they use nitroguanidine and not nitrourea.
PHILOU Zrealone will just H2SO4/Nitrate salt (like dry AN) will yeld k-6 from nitrated NIHT.HCl or not?
Edit: Also K-6 is stable towards hydrolysis, as long as it also contains an urea group, or it is not ?
[Edited on 18-3-2014 by underground] |
Yep that is the reason why.
But in your scheme it is shown that 3 arrows uses Ac2O/HNO3 to give 2 different products...there must be different conditions and NNHT should also
lead to NTNHT with Ac2O/HNO3.
I honestly don't know if NIHT.HCl and H2SO4/NH4NO3 will lead to k-6.
You last question is funny because a few post ago you wrote:
"Compound XIV (1,3,5-trinitro-1,3,5-triazacyclohexane-2-one), a powerful explosive, is stable under normal conditions but decomposes to
1,3,5-trinitro-1,3,5-triazapentane in dioxane and to methylene dinitramine salts in an aqueous medium upon contact with moisture, amines, bases,
alkalis, metals, etc."
And this sentence fully reply to your question... moisture and aqueous imply water ... so k-6 is sensitive to water, bases and acids.
[Edited on 18-3-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by underground  | Quote: Originally posted by PHILOU Zrealone  |
It needs to be tested, but while nitroguanidine is relatively stable towards hydrolysis as its dervivatives NIHT.HCl; the nitrourea and its putative
derivative will be more prompt to hydrolysis... So you will probably get ring opening, decarboxylation to get O2N-NH-CH2-NH-CH2-NH2 wich should be
unstable in HCl and the final product would then be hexamine dihydrochloride and depending on the condition methylamine, dimethylamine, trimethylamine
hydrochloride and NH4Cl. |
So that it is why they use nitroguanidine and not nitrourea.
PHILOU Zrealone will just H2SO4/Nitrate salt (like dry AN) will yeld k-6 from nitrated NIHT.HCl or not?
Edit: Also K-6 is stable towards hydrolysis, as long as it also contains an urea group, or it is not ?
[Edited on 18-3-2014 by underground] |
Yep that is the reason why.
But in your scheme it is shown that 3 arrows uses Ac2O/HNO3 to give 2 different products...there must be different conditions and NNHT should also
lead to NTNHT with Ac2O/HNO3.
I honestly don't know if NIHT.HCl and H2SO4/NH4NO3 will lead to k-6.
You last question is funny because a few post ago you wrote:
"Compound XIV (1,3,5-trinitro-1,3,5-triazacyclohexane-2-one), a powerful explosive, is stable under normal conditions but decomposes to
1,3,5-trinitro-1,3,5-triazapentane in dioxane and to methylene dinitramine salts in an aqueous medium upon contact with moisture, amines, bases,
alkalis, metals, etc."
And this sentence fully reply to your question... moisture and aqueous imply water ... so k-6 is sensitive to water, bases and acids.
[Edited on 18-3-2014 by PHILOU Zrealone] |
This is because of my poor english.
So, the tendency to hydrolyzed really does not sounds good...
NTNHT looks more promising for many reasons (as Motherload said), but i can not found nothing about its properties and its manufacture process
|
|
|
| Pages:
1
2
3
4
..
6 |