| Pages:
1
2 |
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Good experiments suited for winter?
Hello fellow mad scientists, my work decided to lay me off so as of now I have nothing but time on my hands and have been bored to death. That being
said its freezing cold out so I haven't had much reason to go outside to my lab  .
So I'm hoping somebody here can give me some good ideas so I have a reason to go outside and do something useful! .
So I'm hoping somebody here can give me some good ideas so I have a reason to go outside and do something useful!
This being said, what I'm looking for is any experiment\reaction that is beneficial\easier to do at colder temperatures. For example I've already
taken advantage of all the snow outside and my left over pool sanitizer to make a decent stock of chloroform for future extractions. If anybody has
any thoughts on this please post! 
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Using the same NaOCl, hydrazine sulfate is also nice to synth in large batches when it is as cold as it has been in MI recently! Where in MI are you
at? It might be nice to be able to trade some reagents.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Make bromine or nitrate something 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Any reaction as long as it's not endothermic . . . ?
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Pretty much any reaction that would normally require me to go out and buy a bag of ice \ raid my ice maker would be sweet. But anything that requires
extra cold too like that from a dry ice acetone bath. And as for hydrazine sulfate I've already made plenty of that, Bromine sounds fun and dangerous
but I dont have any ampoules  . As for nitrating, my eye did catch a thread for
synthesizing RDX earlier.... and I recently got some formic acid too . As for nitrating, my eye did catch a thread for
synthesizing RDX earlier.... and I recently got some formic acid too 
[Edited on 31-1-2014 by PeeWee2000]
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Making ether is always good for sub-zero temperatures.
Easy and safer to do outside anyway.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Too bad its not cold here in Texas... it was like 20oC today, about room temperature.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Throw some scalding hot water outside and watch it crystallize, like all of those newscasters do. 
Whatever you do, don't set off thermite in the ice without all necessary safety precautions.
Try throwing some sodium on ice. Nothing will happen. Add some liquid water, through, and you can melt ice very easily with the oxidation of sodium,
the exothermic reaction that converts the oxide to the hydroxide, and subsequent dissolution of the hydroxide in the water.
Manganese heptoxide is less prone to explode on a cold day, but it's still dangerous to handle without safety precautions.
Or try some sort of metal-halogen reaction, like Al + Br.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Distilling DCM, ether, or other low boiling solvents perhaps...
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I like a good beef stew with dumplings in winter. 
Seriously, shouldn't this be in miscellaneous or beginnings?
[Edited on 1-2-2014 by blogfast25]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
If you have the experience you could use the weather to your your advantage by doing experiments with HCN.
Or you could try making nitrocellulose which is always fun. I personaly used the cold weather where I live to make a bit of liquid SbH3 so I say that
cold weather can be nice.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I've always wanted to liquefy SO2, but it isn't cold enough here, you might want to try that. (boiling point -10°C.)
[Edited on 1-2-2014 by Zyklonb]
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
hey i am in Michigan too!! lets exchange chemicals and stuff!!!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
No.
The easiest is °
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Hmm... On Thursday it's supposed to get down to -16°C where I live. I hadn't thought of making liquid sulfur dioxide, but I might give it a
try.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Not to continue this topic, but I found what I was looking for: to make the ° symbol, hold down the alt key, and on the #pad type 0176, when you let
go of alt, ° will appear.
Of course I didn't find this randomly. Or Alt+ 248 works too.
[Edited on 1-2-2014 by Zyklonb]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<em>If</em> you're on Windows, and <em>if</em> you have a numeric keypad. Some of us use Linux on notebooks, others use Apple
computers, etc. My suggestion will work on any system.
<a href="https://en.wikipedia.org/wiki/Alt_code#Alternative_input_methods" target="_blank">Alt code</a> <img src="../scipics/_wiki.png"
/>
[Edited on 2.2.14 by bfesser]
|
|
|
Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Chloroform synthesis
|
|
|
killer_lapin
Harmless

Posts: 47
Registered: 23-7-2010
Location: Qc, CAN
Member Is Offline
Mood: No Mood
|
|
You need to be colder than -16 to get liquid SO2, because you have to take consideration of vaporisation enthalpy and heat capacity. I've recently
tried to reflux butane with an ethylene glycol cooler at -20, eventhough butane bp is -0.5C it didn't work so for SO2 it is even less likely.
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Making some liquefied SO2 sounds pretty cool, I've got about 10lbs of powdered sulfur and a dry ice vendor right around the corner, but I dont know
how I would generate the SO2 and capture it, any ideas anyone? Ive got a setup for generating, drying and liquefying NH3 but I doubt it would be
easily adapted to SO2 :|.
Bot0nist thats an excellent idea, tis the season for starter fluid to be on the shelves and hopefully on sale, totally forgot about that!
Bismuthate I'd love to distill some HCN, it really is optimal weather for that, but I dont have any NaCN,KCN,KFeCN etc to generate the HCN from 
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Make a SO2 generator.
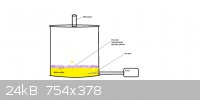
This is what my SO2 generator looks like, I actually use a hand pump, but you get the idea. The SO2 will be rather impure, but
it has worked for me.
Second edit: I will provide much more detail on the making of it in the morning, as well as actual pictures... Burning sulfur is much cheaper than
any of the methods in the link.
Third edit:
Here is my SO2 generator, to run it, I fill it up with about 200-300 grams of rather impure sulfur, melt it, put a fuse in it when it's
lit, close the lid, open the valve, turn on the pump*, it should stay molten from its own heat, although it wouldn't matter either way, as long as you
supply O2, it will continue to burn.
*Or use a hand held pump.
[Edited on 2-2-2014 by Zyklonb]
[Edited on 2-2-2014 by Zyklonb]
[Edited on 2-2-2014 by Zyklonb]

|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
CaCl2 + snow (ice?) is said to reach as far as -50*C, anyone see potential in this?
i recall NH3 having boiling point around -33*C and if SO2 has at -10*C.. then wow
you could potentially drop MnO2 into straight liquid SO2.. its such a pain in the ass to react MnO2 with SO2 :S
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I completely forgot that you can make MnSO4 from Mn and SO2, I was just about to make some with H2SO4, I
think I'll try this now though, thanks for the idea. Sadly I'll have to do this with SO2 gas, not liquid, but surly it will be cheaper
than wasting what little sulfuric acid I have left.
Edit: Misspelled formula.
[Edited on 2-2-2014 by Zyklonb]
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
On a mac, you can do shift, option, 8.
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
cyanureeves
National Hazard
   
Posts: 737
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i am bubbling ammonia from 200 grams ammonium nitrate and 100 grams sodium hydroxide into a quart of 15% janitorial ammonium hydroxide.hardly any gas
is escaping due to the freezing weather we are having and i have no backflow because i purchased a cheap plastic check ball valve off e-bay.later i
will filter it on my mrs. butterworth vacuum flask which i made by drilling a hole on the jar with a cheap diamond hole drill bit also from e-bay.i
got 4 diamond impregnated hole saw drill bits for about 6 dlls.YAY china! and e-bay.come on! global warming.
[Edited on 2-2-2014 by cyanureeves]
|
|
|
| Pages:
1
2 |