thebean
Hazard to Others
  
Posts: 116
Registered: 26-9-2013
Location: Minnesota
Member Is Offline
Mood: Deprotonated
|
|
Potential Anhydrous AlCl3 Synthesis
I had a thought on a simple way of producing anhydrous aluminum trichloride. First I thought of dissolving chlorine in methanol a la this video. After further research it occurred to me that it would be easier and safer to use hydrogen chloride instead of chlorine. Even in
the absence of water, hydrogen chloride can still act as an acid. For example, hydrogen chloride can dissolve in certain other solvents such as
methanol, protonate molecules or ions, and serve as an acid-catalyst for chemical reactions where anhydrous (water-free) conditions are desired.
This was found on wikipedia. I figure if one were to do this they would take a two neck RBF (or find a way to substitute it). In one neck a drying
tube would be placed which would have tubing on the end that lead into methanol with aluminum foil pieces floating around in. The other neck would
have a funnel in it where sodium chloride would be added sulfuric acid in the bottom of the RBF. From my research it seems like a viable method of
producing the invaluable AlCl3. I will hopefully be giving it a go in the near future.
"You need a little bit of insanity to do great things."
-Henry Rollins
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
We have several threads about this already. Peach seems to have found anhydrous HCl in DCM ineffective. I have had moderate success with anhydrous
chlorine gas in dichloromethane. It certainly works...vigorously. Side reactions are the problem. Furthermore:
1) Methanol and AlCl3 react.
2) The bare aluminum metal also reacts with DCM....and most other things. However, it is possible to isolate AlCl3 from the byproducts when using DCM
unlike with protic or polar solvents which give aluminum alkoxides/hydroxides/etc. or stable complexes such as the etherate.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
AlCl3 by chlorine and a chlorinated solvent works very well lbeit it has to be resublimed for purity. The trick is to add a pinch of mercury as this
makes it so much easier for the reaction to start.
This was done with success for a little AlCl3 and quite a lot of SnCl4, perchlorethylene was used as solvent for the simple reason of availability at
the time and the possibility to distill SnCl4 just out.
A little amalgam is claimed to make it possible to generate AlCl3 in benzene for example for a FC in situ by venting dry HCl in. Also the HCl formed
by the reaction will then be reused for making more AlCl3.
If this works - would be great. Never tried it.
Nowadays I would look if AlBr3 cannot fullfill my needs first. Thats far nicer to produce in a similar setup.
/ORG
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
I was thinking this kind of apparatus:
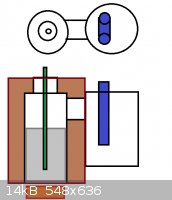
Wool insulated SS vessel with secondary chamber with water condenser circuit on top. Chlorine/HCl gas from the top tube into molten aluminium, which
creates AlCl3 and sublimates it into the cooler and drops to the bottom of the collector. Super easy and can make kilos of it.
[Edited on 16-2-2014 by testimento]
|
|
|
bfesser
|
Thread Moved
16-2-2014 at 06:45 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Organikum  | AlCl3 by chlorine and a chlorinated solvent works very well lbeit it has to be resublimed for purity. The trick is to add a pinch of mercury as this
makes it so much easier for the reaction to start.
This was done with success for a little AlCl3 and quite a lot of SnCl4, perchlorethylene was used as solvent for the simple reason of availability at
the time and the possibility to distill SnCl4 just out.
|
You have evidence/a reference for this?
What does the mercury do?
[Edited on 16-2-2014 by blogfast25]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
@testimonia, after 300 degree C, steel react with chlorine to make volatile chlorides.
@blogfast, mercury amalgamate, effectively disturbing aluminium passivation, making it very reactive. if you got some mercury chloride, you may want
to amalgamate a piece of aluminium rod, and then dry it and expose it to air. You can see the aluminium oxidize with your bare eyes.
I never asked for this.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
It certainly does, I didn't remember to mention to coat the inner core with chlorine-resistant materials, like alumina plaster or even direct silicate
coating or just simply by claying. The tube that should be submerged would necessarily be quartz, but it won't cost more than 5 bucks even in the
draconic's nest I live in.
|
|
|