| Pages:
1
..
9
10
11
12
13 |
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Don't trust anything from ledgards book- it is notoriously full of inaccuracies and dangerous procedures which were poorly researched. You would be
much better off deleting it off your hard drive and using the space for something better. There are much more accurate synthesis described in this
thread.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yeah I made this a couple of years ago using Ledgards book and it is one of the only synthesis i have attempted from it.
I don't use his book as a guide for any explosive synthesis at all now.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
A piece of the product was put in water and the water heated until it melted. Started at 55 Degrees and was fully melted at 60 Degrees.
Very strange, between the melting point of MNT and DNT but nowhere near TNT.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
There is a mirror of the Megalomania website on the Deep Web:
nope7beergoa64ih.onion
I stumbled across if by accident while searching "Astrolite Synthesis"(The procedure was very close to what I had thought it would be) using the
Disconnect Search engine,the default search engine used by the TOR Browser, which can be downloaded here. In case you are wondering,I use TOR because my other browsers are slower compared to it, and I am too paranoid to simply use the HTTPS
version of this forum, especially after the phosgene thread that I posted in Organic Chemistry a while ago.
Just a heads-up, I caught a glimpse of a "cooking" section on the website,though I will not visit it for obvious reasons. I am aware that this post
does not necessarily concern the preparation of TNT,but I am simply posting the link to a mirror of the now-gone Megalomania website.
Edit:I think that this belongs in another thread, I will start a separate thread linking the Deep Web mirror.
[Edited on 10-11-2015 by Agari]
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Someone has already taken the time to copy the whole TNT synthesis by mega on the first page of this thread about three posts down so there is no need
to visit the link.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by NeonPulse  | | Someone has already taken the time to copy the whole TNT synthesis by mega on the first page of this thread about three posts down so there is no need
to visit the link. |
But now we have access to more energetic material syntheses!
Looking at other proposed methods in this thread,we now know which one is the "true" method.
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Instead of SO3 whatever other route, I added 30ml m-phosphoric acid to my acid mix and DNT. Results look pretty good, except the fumes were worse than
normal.
[Edited on 14-3-2016 by Daffodile]

[Edited on 14-3-2016 by Daffodile]
[Edited on 14-3-2016 by Daffodile]

|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Hmm I tried compressing some in foil, and heating lightly. It would just deflagrate, and not detonate at all.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
A few grams TNT or DNT will not detonate from heating confined inside aluminium foil...well maybe if you trow it naked onto a 2000-3000°C molten
metal bath...the Al foil will act as insulator before acting as a heat transfer media slowing down heat transfer to your TNT/DNT and thus it may
vapourize and burn /deflagrate before detonation.
TNT and DNT are relatively insensitive for such low amounts you need a detonator.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
I recrystallized the product from Methanol. Strangely, it crystallized in two portions, which I unintentionally separated when I poured the solvent
off the first, allowing the second to crystallize separately. The first portion (also the larger one, about 3 times the mass) had a melting point of
81.5 degrees C. The second had a melting point of about 73 ish.
I would highly recommend my phosphoric acid route, even though about 30% yield was lost at some point.
[Edited on 15-3-2016 by Daffodile]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Sulfuric acid is just much more common and normally much cheaper I think, but yes other things can likely be used. For small scale I guess cost really
doesn't matter much. The SO3 was just used to prepare 98% sulfuric acid, which works great to produce TNT from DNT, but is actually pretty difficult,
and extremely inefficient, to prepare by simply boiling down lower concentrations of H2SO4 because of loss of sulfuric acid as vapor without proper
distillation equipment, water absorption and the decomposition that takes place at high temperatures/high concentrations.
[Edited on 15-3-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Just this weekend I decided to revisit making TNT and the results were much more satisfying than my first attempt. Once again I used a synthesis
devised by Hennig brand a few pages back where ammonium nitrate is the nitric source along with good old drain cleaner of around 93% if my density
measurement was correct. However this time I allowed for 20% excess unlike my 1 st attempt. I made up the nitrating mixture and let it sit in the
freezer for an hour while I attended something else. This time I was much better equiped for the task since last time I just used a beaker with hand
stirring.
The nitration mixture was charged into a 1l RBF with two necks, and a large oval stir bar dropped in, a thermometer adapter in one mouth and a
pressure equalising addition funnel to the other and turned the tap to slowly drip 50g toluene into the reaction mixture at a rate of a drop a second
slowing occasionally to let the temp not rise too fast and go above 55 yet. For the last 10 mls I just let it rise and 10 mins after the last drop the
funnel was switched for a condenser to reflux. Heating was started cautiously and slowly to get up to 80c where it was held for 3 hours with a brief
period where it crept up to 90 without me realising. This was soon fixed though. I let the vessel cool a bit and transferred the RBF contents into a
500 ml beaker to cool completely and solidify.
A funny thing happened though, the DNT did not solidify and had me worried that I had somehow effed it up even after an hour n the fridge so I decided
to use a syringe to syphon the layer into water and this is where it decided it set solid slowly like the hot ice effect. Scooped this out and
quenched the acid into water precipitating a little more DNTthis was placed with the cake into about 600 ml distilled water and heated to melt it
while strong magnetic stirring was applied to wash it. The DNT cake was allowed to set cool and fished our out of the beaker, rinsed off and dried for
weighing. It weighed 75.1 g and was crystalline with small needles and had very little MNT smell to it. The first batch I made smelt quite a bit like
MNT and this one does not at all. There was pretty much no smell during nitration either except when I switched to the condenser so it was well
contained in the chosen reactor. I will be completing the final nitration in the next week or so and am actually considering making oleum with some
phosphorus pentoxide that I have around, that is if I find the time to otherwise it's just going to be a standard nitration mix of concentrated mixed
acids.
T
  
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Nice looking setup! Really good that you are using reflux! I think your yield should be closer to 100g of DNT from 50g of toluene however.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Thanks! it really helps when you have the right equipment for the job.
I did see you got 100g DNT in your earlier post and i expected similar but i guess there was more impurities in my batch that were removed during the
washing stage and also i set the cake up onto a few layers of paper towel to wick out moisture and upon leaving it overnight the paper towell was
laden with an oily exudation from the cake and i suspect a lot of the extra weight was lost there. maybe there was losses as a result of the extra
hour of reflux but that seems unlikely.
but anyway i'm still quite pleased and i must say as far as a synthesis for energetics goes this is quite easy to do and required minimal
supervision- just a regular eye on the thermometer and wait. all in all a much better outing than my first attempt
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have a suspicion that only one mole of nitrate per mole of toluene was added instead of the required two moles of nitrate per mole of toluene. I
added the nitration mixture to the toluene while you did the opposite, I think both can work fine though. Unlike esterifications, aromatic nitrations
are not equilibrium reactions, so if the temperature and rate of toluene addition were in line nearly all of the first half of the added toluene would
be converted to DNT and most of the rest of the toluene addition would be left un-nitrated. The un-nitrated toluene would act as a solvent as well
which probably lowered the yield also. This is my theory anyway.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ManyInterests
National Hazard
   
Posts: 837
Registered: 19-5-2019
Member Is Offline
|
|
https://youtu.be/uwASaAqyuAk
This thread has been a goldmine of knowledge about making TNT. It wasn't something I thought I could make, but after reading this thread and watching
the above video that I linked, I am seriously considering making some. I already got pure toluene and sodium bisulfite (metabisulfite actually, but it
becomes bisulfite when added to water, which is what is needed anyway).
I've seen so many methods listed that I will just ask for advise using the video as a reference. in the video they use around 200ml of H2SO4 (I will
make sure the concentration is as close to 98% via boiling as possible) and 115g of ammonium nitrate for both steps, and 50g of toluene. In the video
he mixes the nitrating mixture first and then cools it to room temperature before adding the toluene dropwise.
The actual process is not what I am curious about, since it seems quite straightforward. What I am concerned about are the reagents. He nitrates the
toluene to DNT via ammonium nitrate and H2SO4, and then he says that a 'second' nitrating mixture is made for the DNT to TNT step. I obviously assume
that he uses another 200ml of H2SO4 and another 110.5g of ammonium nitrate (I will be using a slight excess of both in my case). Would this work?
because if that is the case, it would make making TNT a far more simple and economical process than other energetics that often require very
concentrated HNO3, such as RDX.
Also for the nitrate salt. Is the ammonium ion preferable to using something like sodium nitrate or potassium nitrate?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I never nitrated DNT with a salt, but ammonium nitrate gives a nicely stirrable mixture while KNO3 doesn't mix well with sulfuric. NaNO3 is hard to
get dry, probably also doesn't mix well.
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ManyInterests  | https://youtu.be/uwASaAqyuAk
This thread has been a goldmine of knowledge about making TNT. It wasn't something I thought I could make, but after reading this thread and watching
the above video that I linked, I am seriously considering making some. I already got pure toluene and sodium bisulfite (metabisulfite actually, but it
becomes bisulfite when added to water, which is what is needed anyway).
I've seen so many methods listed that I will just ask for advise using the video as a reference. in the video they use around 200ml of H2SO4 (I will
make sure the concentration is as close to 98% via boiling as possible) and 115g of ammonium nitrate for both steps, and 50g of toluene. In the video
he mixes the nitrating mixture first and then cools it to room temperature before adding the toluene dropwise.
The actual process is not what I am curious about, since it seems quite straightforward. What I am concerned about are the reagents. He nitrates the
toluene to DNT via ammonium nitrate and H2SO4, and then he says that a 'second' nitrating mixture is made for the DNT to TNT step. I obviously assume
that he uses another 200ml of H2SO4 and another 110.5g of ammonium nitrate (I will be using a slight excess of both in my case). Would this work?
because if that is the case, it would make making TNT a far more simple and economical process than other energetics that often require very
concentrated HNO3, such as RDX.
Also for the nitrate salt. Is the ammonium ion preferable to using something like sodium nitrate or potassium nitrate? |
With a big enough excess of acids you can make TNT straight from toluene in a single nitration. And yes, ammonium nitrate works fine.
Just be aware of the toxic and carcinogenic properties of TNT and more importantly its lower nitrates. Not something to handle carelessly in terms of
skin contact, inhalation or ingestion. like you could many other compounds like ETN for example.
|
|
|
ManyInterests
National Hazard
   
Posts: 837
Registered: 19-5-2019
Member Is Offline
|
|
I am well aware of the toxicity of TNT. I was quite cautious with a lot of other dangerous material and I plan on applying the same caution to TNT as
well.
The reason why I was asking about ammonium nitrate and not a nitrate salt is because even though I can make WFNA (I need to make it a few more times
to make sure that I can make it consistently. The last I did it, I made some with a specific gravity of 1.52 at 15C...), it takes a lot of nitrate
salt to make that much WFNA. This is why if I wanted to make picric acid again, I would use sodium nitrate and not bother with trying to make nitric
acid since I would need much more nitric acid than I would in nitrate salt.
| Quote: | | I never nitrated DNT with a salt, but ammonium nitrate gives a nicely stirrable mixture while KNO3 doesn't mix well with sulfuric. NaNO3 is hard to
get dry, probably also doesn't mix well. |
I didn't find NaNO3 to be difficult to dry. Putting it in the oven for a few hours and stirring it around at around 150C will make it dry as a bone. I
don't know about its mixability in a nitrating mixture (haven't tried that) but I do agree that KNO3 forms a thick mixture and is annoying.
I can make ammonium nitrate with the sodium bisulfate, ammonia, and other nitrate salt (sodium nitrate is currently my favorite since it is much
cheaper to make than other nitrate salts).
| Quote: | | With a big enough excess of acids you can make TNT straight from toluene in a single nitration. And yes, ammonium nitrate works fine.
|
How much of an excess are we talking about here? In the video the total amount of reagents he used would be 400ml of H2SO4 and 221g of ammonium
nitrate. If I put in 500ml of H2SO4 and 300g of ammonium nitrate, would it be sufficient for fully nitrating 50g of toluene? I need some kind of
formula or stiochiometry here if you can provide it. Please explain the science!
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
At the warmer temperatures you run a toluene nitration ammonium nitrate is not too viscous to stir and I'd guess that's also true with sodium nitrate
though I never tried it.
You really don't need fuming nitric acid and oleum to get it to full nitration or anything special. It can actually be prepared in a single nitration
with 94% drain cleaner grade sulfuric acid and ammonium nitrate at the expense of wasting some potential of the reagents.
It's industrially more efficient to prepare it in multiple steps but you're making grams, not tons.
Just try a small batch with an excess of SA/AN mixture. Add toluene dropwise with good stirring, let it go for a while then bring the temperature up
slowly and let it stir for an hour or so. You'll be left with TNT.
|
|
|
ManyInterests
National Hazard
   
Posts: 837
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by OneEyedPyro  | At the warmer temperatures you run a toluene nitration ammonium nitrate is not too viscous to stir and I'd guess that's also true with sodium nitrate
though I never tried it.
You really don't need fuming nitric acid and oleum to get it to full nitration or anything special. It can actually be prepared in a single nitration
with 94% drain cleaner grade sulfuric acid and ammonium nitrate at the expense of wasting some potential of the reagents.
It's industrially more efficient to prepare it in multiple steps but you're making grams, not tons.
Just try a small batch with an excess of SA/AN mixture. Add toluene dropwise with good stirring, let it go for a while then bring the temperature up
slowly and let it stir for an hour or so. You'll be left with TNT. |
Yeah, in the video the guy used 50g of toluene, I will not be using more than 100g at a time (and even that is quite a lot). Industrial production is
an entirely different beast. Those factories, even older factories during WW2 with fairly primitive equipment compared to today still churned out
unfathomable tons.
So I am still wondering what an excess would be, and if I need more than a 2 stage process. I still need a bit of an idea, but the stuff I have in
mind is a little over double the amount the guy used in his video. Without more information, that is all I have going in my mind. All information I
have on TNT production comes from this thread. I might need to open up an Urbanski book at this point, but basically most of what they describe is
industrial processes.
Edit: Now that I've learned about how much more liquid ammonium nitrate is in sulfuric acid. I might try to make ETN or PETN syntheses with it. Since
one of the first attempts posted on this forum who made ETN did so with ammonium nitrate and got a fairly good yield, I might do that instead of
making nitric acid, since it will be more economical that way. I don't know about how much ammonium nitrate I would need with sulfuric acid to make
PETN, so I'll need more research on that.
Edit2: Actually on second thought, maybe a 2-stage process might still be better for my purposes.
[Edited on 8-9-2023 by ManyInterests]
[Edited on 8-9-2023 by ManyInterests]
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ManyInterests  | | Now that I've learned about how much more liquid ammonium nitrate is in sulfuric acid. I might try to make ETN or PETN syntheses with it.
|
PETN can indeed be obtained from mixtures of sulfuric acid and nitrate salts. But due to the ease of esterification, it is still more profitable to
synthesize it using sulfur-nitrogen mixtures, since even with 56% nitric acid a yield of >90% is achieved.
But regarding production through nitrate salt.
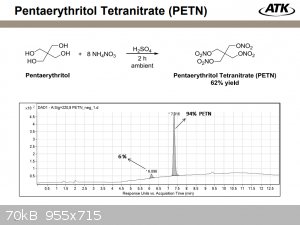
The ATK company obtains it with a yield of 62% through ammonium nitrate (8 mol) and a certain amount of sulfuric acid within 2 hours. I'm not sure if
this is beneficial...
You can first obtain pentaerythritol sulfate by dissolving it in 6-7 parts by weight of concentrated sulfuric acid, then usually (this method is
called a two-stage method) add 10-12 parts of nitric fuming acid (not necessarily white). Here, in theory, you can replace it with a mixture of
sulfuric acid and nitrate salt. Just recalculate the proportions. Someone even managed to get the same yield of 80-90%.
Then everything is the same, transesterification at 55-60°C, dilution with water, neutralization, boiling in a 1% soda solution and then
recrystallization from acetone from a soda solution or grinding with chalk (if there is no acetone).
But in general, by the way, this is off-topic, I’m not sure that another explosive can be discussed in the topic about TNT. In general, by the way,
me need to check with the administration.
[Edited on 8-9-2023 by DennyDevHE77]
[Edited on 8-9-2023 by DennyDevHE77]
|
|
|
ManyInterests
National Hazard
   
Posts: 837
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | PETN can indeed be obtained from mixtures of sulfuric acid and nitrate salts. But due to the ease of esterification, it is still more profitable to
synthesize it using sulfur-nitrogen mixtures, since even with 56% nitric acid a yield of >90% is achieved.
But regarding production through nitrate salt.
The ATK company obtains it with a yield of 62% through ammonium nitrate (8 mol) and a certain amount of sulfuric acid within 2 hours. I'm not sure if
this is beneficial...
You can first obtain pentaerythritol sulfate by dissolving it in 6-7 parts by weight of concentrated sulfuric acid, then usually (this method is
called a two-stage method) add 10-12 parts of nitric fuming acid (not necessarily white). Here, in theory, you can replace it with a mixture of
sulfuric acid and nitrate salt. Just recalculate the proportions. Someone even managed to get the same yield of 80-90%. |
There is another thread about PETN or close, we can bump that up if you want (I wrote about it in the RDX thread). I just do want to make this one
single comment here.
OK so making PETN with nitric acid is better. But I do have one question about it: What could have made my yields go bad in the one time I tried it? I
used 88g of PE (98%, it was technical grade), but I ended up with only about 122 grams of the stuff. In all videos I've seen an literature I've read I
should have ended up with 166 or a bit more.
I used 290ml of 90% HNO3 (purged of all NO2 contamination by blowing dry air through it) and 170ml of H2SO4. I froze the beaker in a solid block of
salt water ice (as I normally do. The ice always melts to a very large degree during the additions) and I added the PE gradually, but I did not time
it. During the addition I did notice some yellowing happening. There were no major temperature spikes and temperature was below 20C during all the
additions. This yellowing was concerning to me as I thought it was destroying my product and this is what wanted to try to hurry things along. After
the addition, I did let the temperature rise to room temp, but I kept returning it to the ice water bath. I wasn't sure if it was gonna stay at 25C or
not. After a while of doing this (not timed either) I decided to move onto the heating step. As I was heating it, I did notice the yellowing get
darker and I did see some very faint brownish red fumes start to form. To me, this was the sign of a runaway and I decided not to take any chances. I
cut my losses and crashed everything into a bucket of cold water.
The cleaning was done as normal. lots of water, followed by bicarbonate solution, followed by more water, then let it dry. After wish I recrystalized
it in acetone. It was quite an adventure since the the liquid during recrystalization turned orange!
What could have caused all this and had such an effect on my yield?
I promise, you can bump up another thread if you don't want to continue it here.
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 279
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Brining up the temperature too much toward the end of nitration isn't necessary with PETN, it's rather easily brought to full nitration by bringing it
up to 25-30C in warm water for the last half an hour.
In my experience PETN is fairly tame and not prone to runaway IF constantly/thoroughly stirred in a cold water bath and the PE additions are done in
a few steps with a couple minutes in between to make sure the temp isn't climbing which for me it never has.
I would guess the NO2 evolving when you heated it was likely decomposition of your PETN, that's probably where your yields went.
|
|
|
ManyInterests
National Hazard
   
Posts: 837
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by OneEyedPyro  | Brining up the temperature too much toward the end of nitration isn't necessary with PETN, it's rather easily brought to full nitration by bringing it
up to 25-30C in warm water for the last half an hour.
In my experience PETN is fairly tame and not prone to runaway IF constantly/thoroughly stirred in a cold water bath and the PE additions are done in
a few steps with a couple minutes in between to make sure the temp isn't climbing which for me it never has.
I would guess the NO2 evolving when you heated it was likely decomposition of your PETN, that's probably where your yields went.
|
Duly noted. Next time I will simply allow the PETN to stay at room temperature for a while before simply crashing it in a large amount of ice cold
water.
I will also use an excess of around 75% nitric acid and go as strong as 90%. I am hoping to have the yield that others have had (2.2g PETN per 1g PE)
than the poor yield I got.
I will also time it and try to not to worry too much about the yellowing. It is probably not as bad as I made it out to be.
|
|
|
| Pages:
1
..
9
10
11
12
13 |