copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
making bromine water
In this video http://www.youtube.com/watch?v=AEQhlUQzlrM you use potassium bromide, hydrogen peroxide, and hydrochloric acid to make bromine water. My question
is wouldn't there be potassium chloride from the potassium bromide? Thanks
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Bromine water, made in that way indeed contains a lot of impurities. Potassium ions, chloride ions, but most likely also acid and some excess bromide
or excess hydrogen peroxide.
If you want to make pure bromine water, then you need to drive off the bromine vapor from this aqueous solution and lead these vapors through clean
water.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
woelen could i do that without a distillation setup? Because i dont have one.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
You can use glass tubing to and stoppers with holes to direct the bromine vapor if you have some.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
ok bismuthate but wouldn't the bromine corrode the stopper?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Make sure you do this outside because the bromine vapors are toxic and this method produces considerably more loss, but is cheap to do. Just pour the
solution from the reaction into a beaker and place a funnel which just fits inside of it in it, the closer it is to the sides, the less loss there
is, stem up. Then connect some clear tubing to the stem of the funnel and another funnel which is place into another clean beaker with some distilled
water. Make sure the funnel in the collection beaker is just below the water line so less bromine escapes. I would recommend putting the collection
beaker into an ice bath to ensure maximum condensation and yield of bromine water.
Edit : Oops someone got there before me.
[Edited on 22-3-2014 by gdflp]
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
gdflp are you referring to glass or vinyl tubing?
[Edited on 22-3-2014 by copperastic]
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
By the way, I have a distillation flask made up of a Florence flask with a tube coming off the side.
(http://www.hometrainingtools.com/distilling-flask-with-sidea...)
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
If its a one time thing you could use vinyl just be aware that it will get stained. Glass is definitely a better choice. Vinyl is generally bad for
anything but water (great for cooling condensers) Especially for vapors which may be hot and weaken its structure.
Rubber tubing could work too.
[Edited on 3-22-2014 by numos]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
PVC is actually remarkably chemically and thermally stable. It has no unsaturation, for instance. Against hot bromine it is not ideal but it will
resist better than you might think.
As regards rubber tubing, many types of it are made of either natural rubber or styrene butadiene rubber based mixtures. Both contain large amounts of
unsaturation (double bonds) which are ideal point of attack for things like halogens or ozone. Mere prolonged heat will cause these rubbers to harden,
due to crosslinking of the double bonds. Tubing made of ethylene propylene copolymers based mixtures is much more heat and chemically resistant but
more expensive.
As always, the proof of the pudding is in the eating...
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
so blog fast do you think it might work against bromine?
Also would it work against chloroform because i might make some of that.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I'd like to share my experience with preparation of bromine water. Sure, it can be done by many different ways. And it is just one of them. My goal
was to make the reasonable pure bromine water and small amount of Cl2 as well as ions Cl- are acceptable impurities.
Reagents:
KBr, H2SO4 (95%), NaOCl (12%)
Some important additional equipment:
3M gas mask (I use ABEK1 filters and they block bromine vapors very well if you follow this procedure), a fan, spray bottle with 5-10% NH3 solution,
a silicon grease for all joints.
Procedure.
The first step is making HBr. I use the method from Vogel "Organic chemistry".
Dissolve 120g KBr in 200 ml of water. The dissolving is endothermic so by using hot water I can do it fast. Put the solution into 500 ml flask and
then put the flask in ice-cold water bath and slowly add 90 ml of concentrated H2SO4 (I used 95%). Usually I make this procedure in the following
apparatus:

With each drop of H2SO4 a cloud of HBr is forming above the solution layer so this apparatus allows to keep it inside until it dissolves in the
solution.
After all the acid is added decant the solution (while still cold) from KHSO4 crystals.

KHSO4 is a useful byproduct

if you need it then mix them with ice-cold water, do vacuum filtration and wash once with ice-cold water.
Getting pure HBr from the decanted solution requires distillation but for the preparation of bromine water it is unnecessary because it will be
distilled in any case.
So, I assemble the following apparatus which allows me to make the HBr oxidation in house.
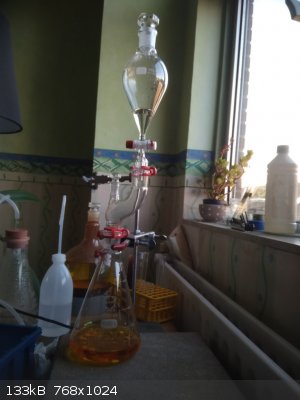
Pour 155 ml of 12% NaOCl into dropping funnel. Put the HBr solution from the previous step into 1 L Erlenmeyr flask, put some boiling chips inside
(after forming bromine fumes it will be harder to do). Grease all connections to keep all bromine fumes inside the apparatus.
Add NaOCl from dropping funnel. The reaction is not exotermic neither develops any pressure. You can see that the bromine fumes are stopped (absorbed)
by the NaOCl in the top part of apparatus, so NaOCl works both as oxidizer and absorbent and simple not pressure-equalized funnel can be used - no
bromine fumes can escape the NaOCl solution.


After all NaOCl is added the bromine is still not formed as a separate layer. It is possible because there is some equilibrium which prevents its
formation in large quantity at this stage.
The rest do outside.

This is a distillation apparatus I use. The fractionating column is used to minimize the bromine water contamination with acid anions. I use a tap
water for the cooler but receiver flask I put in ice water. Then I connect the gas output from the receiving flask with PTFE hose to a wash bottle
which is also immersed in the ice water. Then I use 2 bottles as a two-way trap and I put 200 ml of 6M NaOH solution inside one of them. The output of
the trap could be safely left as in the picture - no bromine fumes can escape the system by it.

The first fraction is Bromine which pass at 52C . Don't stop distillation after that. Just stop water in a cooler and allow a steam of water to absorb
bromine fumes in a flask, column and condenser until the point the distillate enters the receiving flask.

Start water in the cooler again because otherwise heating of the receiving flask by water steam will cause the evaporation of already collected
bromine. This procedure distills water on top of bromine.

Stop the distillation of water when you like but before the temperature reaches 101C, otherwise some acid will be distilled.
The next step is disassembling and it is very important one. Because the distillation of bromine fraction was followed by a water one it is not so big
problem if you work outside, but use a gas mask.
When the system is cooling bromine vapors are pulled back into condenser, column and the Erlenmeyer flask. I use vacuum to clean the system:


(Possible it's better to use this order:
1. Apply vacuum to the end point (hose from the trap)
2. Open stopper on top of the column allowing all fumes pass through the trap
3. Cool the Erlenmeyer flask
This order of operations should prevent pulling bromine fumes back to the condenser, column and Erlenmeyer).
For detaching receiving flask use a fan to pull a cloud of vapors until you stop it (use a silicon grease on a stopper).
Fill everything with water to remove all fumes from all components outside.
So, it is bromine and some water on top of it.

When doing that procedure I accidentally dropped a receiving flask (these clumps are a scrap really) so half of water and some drops of bromine was
lost (more water because I used 500 ml round flask as a receiver and it keeps this quantity of bromine when falls). I used NH3 spray which stops fumes
instaneously (convering them into clounds of NH4Br), then NaOH and some metabisulfite. It was smell after that but the spillage was quickly
neutralized.
I put the flask into old (not working) fridge in a camera where I keep ammonia and ammonium carbonate. I spread some carbonate on the bottom which I
think give enough NH3 fumes inside the camera to neutralize Br fumes which can escape. Possible I will find another solution in case this will not
work.

I will pour a new water on top of bromine each time I will use it, so this small quantity of bromine with water can serve as a bromine water source of
much bigger quantity than can hold this flask.
I will try to distill over HBr from the rest of the solution. The quantity of NaOCl I used is enough to oxidize only one half of it. But I used half
quantity intentionally because I think it minimizes the amount of byproducts.

[Edited on 5-10-2019 by teodor]
[Edited on 5-10-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Nice synthesis of bromine. Bromine water cannot be stored indefinitely. I once made bromine water (just Br2 and water, no other compounds in it).
After a year or so, the liquid had turned colorless, the cap was broken and only an acidic solution was left. I think that slowly, the bromine reacted
with water, giving HBr and O2. The O2 most likely caused pressure buildup, which destroyed the cap. The corrosiveness of the Br2 itself also helped
destroying the cap.
Later, I myself made bromine quite a few times and I also used distillation.
Now I store my bromine in ampoules, which I sealed with a propane burner. I used 10 ml ampoules for storing all bromine I made. It is 100% sealed, no
loss of bromine at all, no smells, nothing. They also make nice element samples. These ampoules already are several years old. Glass ampoules keep
indefinitely, no caps which get corroded. If I need bromine, I can take a single ampoule, break it and use that little amount of experiments, while
keeping the other ampoules closed. I still have almost 100 ml of Br2.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Thank you.
I checked in Vogel "Qualitative inorganic analysis" the method of Bromine water preparation:
"Shake 1 ml liquid bromine with 100 ml water. Ensure that a slight excess of undissolved bromine is left at the bottom of the mixture. The solution
keeps for 1 week"
I was very curious why it deteriorates. I thought about disproportionation to HBr and HOBr but it couldn't explain that. So, the oxidation of water
(with evolution of O2) is very interesting and useful information about the reason why it should be prepared fresh.
How do you think, will the solution of Br2 in concentrated HCl be more stable?
May be I will try ampoules one day, but for now - I have no equipment for that (a propane burner). I have a dedicated freezer for chemicals, so I
think I will put the rest of bromine there.
UPD: yes, really, after 24 hours it was already some pressure inside. I pipetted out the bromine and put it in a freezer. While I manipulated with the
rest (water and small amount of bromine) the corck shooted out. Possible the reason was ammonia vapors outside the flask or shaking the mixture, or
some organic matters flying around and found their way inside the flask. So, my bromine water is really unpredictable, possible the same way as
concentrated H2O2. Will try to consume it as fast as possible.
[Edited on 6-10-2019 by teodor]
[Edited on 6-10-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, some chemicals or mixes simply cannot be kept around safely for a long time. Bromine water is one of them. At the time, I did not know, but I
found out the hard way. I also learned from that exprience. Now I only store pure chemicals and with every chemical I purchase I first check how it is
on storage.
In the past I had bad experiences with
- bromine water
- ammonium peroxodisulfate (decomposed, cap broke, became a wet mass without any oxidizing power)
- calcium hypochlorite (container ruptured, many electronic items, stored nearby, were completely corroded, cost: 1000+ euros)
Now I do not have these chemicals anymore and I do not intend to replenish my stocks of them.
These chemicals cannot even be stored safely in glass ampoules, because they are unstable. Storage in a glass ampoule eventually will lead to rupture
of the ampoule, due to pressure buildup. On the other hand, some very nasty chemicals I now store safely in glass ampoules (Br2, PBr3, PBr5, PCl3,
PCl5, POCl3, SOCl2, SO2Cl2). Some of these I made myself. They make interesting display samples!
[Edited on 7-10-19 by woelen]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by woelen  |
<snip>
in glass ampoules (Br2, PBr3, PBr5, PCl3, PCl5, POCl5, SOCl2, SO2Cl2). Some of these I made myself. They make interesting display samples!
|
You have mentioned these before and have shown photos of some. I would love to see a pic of all of them lined up. 
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
For such requests I will make some time  . . . somewhere this week. . . . somewhere this week.
My extreme corrosives gallery 
Actually, these chemicals are quite funny in some way. They are extremely corrosive and handling them requires great care. At the same time, when
released in the environment, they are quite benign and just add some acidity, which quickly is neutralized. They end up as phosphites, phosphates,
sulfates, chlorides or bromides, all of them being benign. When you clear your pipes with drain cleaner you add acids to the environment in much
larger quantities.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I am also waiting for your photos, woelen.
Some additional experience with bromine water:
1. The reason why I got a stopper shoot from a flask was a temperature change. It seams that the staff is very sensitive to that, so if you have your
water prepared at 10-15 C, stoppered and then put my palm on the flask wall it is enough for pressure to be built in 10-20 secs. When working it is
better to hold the flask by a neck. But 3 days of storage in cold didn't develop pressure.
2. Good shaking for bromine water preparation is essential. It seams bromine doesn't dissolve in water by diffusion in any good extent (I think
because of formation some other compound in a layer between water and bromine). So, after several hours it will be sitting on the bottom. But after
one minute of shaking it will be dissolved completely.
3. To remove bromine vapors from a flask it is enough to poor a little of some reducing agent solution and shake. The brown vapors disappears very
quickly. I tried it with SnCl2 solution but think other staff will work as well.
[Edited on 10-10-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Here follows a picture of most of my ampouled nasties. These make nice display samples, I do not use them for chemical experiments.

The small left-most ampoule is PBr5, with a little PBr7 in it, and the one besides it is pure PBr5. The others have their formula on the ampoule.
[Edited on 22-10-19 by woelen]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
The solid ones look harmless, especially PCl5. Is it really so nasty? 
SnCl4 is something I want to make. But didn't get idea yet of the whole process, from start to finish, how to make it safely.
So, nice collection. Good picture of achievements. How many of them did you synthesize by yourself?
[Edited on 22-10-2019 by teodor]
[Edited on 22-10-2019 by teodor]
[Edited on 22-10-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
PCl5 and SO2Cl2 are the nastiest of the entire set. They are extremely corrosive and it is nearly impossible to find a container in which they can be
stored indefinitely. This is due to the combined oxidative power of these reagents and their general corrosiveness.
I made PBr3 myself and from that I made PBr5 and the PBr7/PBr5 mix. Making and isolating pure PBr7 is hard, it easily loses Br2. PI3 I also made
myself. This is very easy, just dissolve I2 in CH2Cl2 and add red P. The solution turns red and on careful distillation of the CH2Cl2 the PI3 remains
behind. The reaction is easier with CS2 (more I2 can be dissolved in that), but CS2 is harder to get your hands on.
I made POCl3 from PCl5, simply by adding a little water (done outside, very slowly, it produces a lot of HCl). This reaction runs remarkably cool. The
HCl escapes as gas. If too much water is added, then the reaction runs very hot, due to dissolving of HCl in water, which is very exothermic. I
distilled the POCl3 to make it perfectly colorless. The material I obtained from the reaction was sligtly yellow/greenish.
PCl5 and PCl3 I had a one-time opportunity to buy them. Normally they cannot be purchased where I live. SO2Cl2 and SO2Cl(OH) can be purchased where I
live. SOCl2 on the other hand is not available as easily. SO2Cl2 and SO2Cl(OH) are very corrosive, but their environmental end-products are non-toxic,
and they cannot be easily used for making chemical weapons. SOCl2 is a precursor for certain chemical weapons and for this reason it is not easy to
get it.
|
|
|