| Pages:
1
2 |
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Diisonitrosoacetone
I've seen no explosive properties reported for diisonitrosoacetone, but it is a precursor to a new "insensitive high explosive", the reference is the
only hit on google for "dioximinoacetone" but I cant open it from here. Its also used to detect the "G-agents" (sarin, tabun etc.) turning
clear→magenta in its presence (patent US2867509), off topic but I have more info on that if wanted.
It looked interesting enough to try, with both oximes and ketones capable of being converted to explosive groups and no exotic precursors. I got to it
by treating citric acid with sulphuric acid, forming acetonedicarboxylic acid then treating this with NaNO2 converting it to diisonitrosoacetone.
<center><img src="http://www.sciencemadness.org/scipics/axt/dnastructure.jpg"></center>
<I>Experimental</i>: 50g citric acid was added to 250g chilled (-10°C) 98% sulphuric acid, keeping the temperature below 5°C. The citric
took a few hours to dissolve and evolved some bubbles. After its all dissolved it was removed from the fridge and allowed to sit in the sun (35°C
outside). On warming the solution foamed up releasing CO as the reaction commenced. It was stirred in a modified drill press to help with the release
of the gasses and keep temperature consistant, this took a couple hours. Once the foaming had stopped 200g in ice cubes was added to the solution and
once dissolved was chilled in the freezer to -10°C and quickly filtered through a cotton sheet. It was then pressed to remove some of the liquid.
Yield was a poor 12g of wet crystals of acetonedicarboxylic acid. This synth is an adaption of the one from orgsynth. Orgsynth gives a better yield
with the use of oleum. It would also be a good idea to dehydrate the citric before use (I didnt).
The acid wet acetonedicarboxylic acid was dissolved into 20ml of water and cooled to near freezing, into this solution was poured slowly, with
stirring, another solution of 6.5g NaNO2 in 12ml water. after the solution had stabalised it was but into the freezer and chilled. A precipitate was
filtered for a measly final yield of 4g of dry diisonitrosoacetone.
The product obtained seemed explosive in itself, igniting easily and flaring up with a smokeless soft orange flame simular to nitrostarch, this is
shown in the picture/movie below. Its metal salts (or other derivatives?) are likely more interesting. However the "G-agent indicator" patent refered
to above mentions the Na & NH4 salts are unstable, quote "decompose violently at 120°C". I dont know how likely a peroxide is, but it would be a
killer explosive if you could also join the oximes achieving CO balance, there are examples which suggest this may be possible.
<center><img src="http://www.sciencemadness.org/scipics/axt/dna.jpg">
<a href="http://geocities.com/roguemovies7/">MOVIE</a></center>
[Edited on 9-12-2005 by Axt]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Good work!
It's a shame the yield is so bad though.
Could you please point out where you got the reaction from acetone dicarboxylic acid with NaNO2 to the dinitroso acetone (which is a bit misleading,
because really it is an oxime)? I would be interested to see the reaction mechanism - i.e. I am not clear about what happens to the remaining COOH,
which is seemingly replaced by the =N-OH.
Anyway, I had a look for that publication where the the diisonitroso acetone is used as a precursor (see attachment, I am not sure whether it accepts
200kb attachments).
Have a look, it gives the routes/preps for a large variety of heterocyclic HE's, which are often derivatives from the bases of DNA, i.e.
pyrimidines and purines.
[Edited on 7-12-2004 by chemoleo]
Attachment: Studies of heterocyclic exp.pdf (191kB)
This file has been downloaded 2428 times
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
You could well get a better yield with a different precedure, its only one attempt at it. Theres a reference for using H2SO4 (without SO3) at the
bottom of the <a href="http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?print=1&showprint=1&prep=cv1p0010">org synth.
page</a> which would be good to get hold of. I just used an excess of H2SO4, where its likely most of the yield remained in solution.
The diisonitrosoacetone synth was pulled from "preparation of organic intermendiates" where its described in the "diaminoacetone
dihydrochloride" synthesis. I think this is on the FTP as a djvu. Why they call it "diisonitrosoacetone" I dont know, but thats whats
given in the majority of references, who am I to change it!
I changed the structure to what it should be! I must have drawn it 50 times and I get it wrong now 
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
If you still have some of the DINA left, and some hydroxylamine on top of it, maybe it might be interesting to react the DINA with it - it should form
the triply substituted derivative, i.e.
HON=CH-C(=NOH)-HC=NOH - unless it does something quite weird and unpredictable. You can get your hydroxylamine from the respective sulphate with
Ba(OH)2.
Anyway, this should have a higher energy.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I dont have anymore, nor hydroxylamine. But it would be interesting, the trioxime dissolved in ether, bubble in N2O4 converting it to hexanitropropane
 . Well probably not, but CH3-C(=NOH)-CH3 goes to 2,2-dinitropropane when
treated with N2O4. . Well probably not, but CH3-C(=NOH)-CH3 goes to 2,2-dinitropropane when
treated with N2O4.
[Edited on 8-12-2004 by Axt]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Do you have any data on the toxicity and carcenogenity of this compound? Nitriso compounds are usually carcinogenic.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
No, I havnt seen any mention of its toxicity. Its not really a nitroso (<- thats what you mean isnt it), so cant really be compared to them, N-N=O
supposedly being the carcinogenic group. Unless you know something about "nitriso" that I dont!
Heres its original synth:
| Quote: | Extract from "Preparation of Organic Intermediates" (Shirley, 1951)
CO(CH2COOH)2 + 2 HNO2 -> CO(CH=NOH)2 + 2 CO2 + 2 H2O
Koessler and Hanke, J. Am. Chem. Soc., 40, 1717 (1918)'; Pechmann and Wehs- arg, Bet., 19, 2465 (1886).
A solution of 50 g. of acetonedicarboxylic acid (crude, containing some sulfuric acid) [Org. Syntheses Coll. Vol. 1, 11 (1941)] in 100 ml. of water is
stirred and cooled in an ice bath while a concentrated aqueous solution of 30 g. (0.44 mole) of sodium nitrite is added drop-wise. The resulting
mixture is acidified by the slow addition of
dilute sulfuric acid with continued cooling and stirring. The precipitated diisonitrosoacetone is collected by filtration and washed with water. The
yield is about 50%. The product may be further purified by recrystallization from methanol. The pure product melts at 143-144 ° .
|
Heres an example of a dioxime peroxide bridging a ketone group. Extract from "The Furoxans" J. V. R Kaufman & J. P. Picard. Picatinny Arsenal,
Dover, New Jersey (1959).
<center><img src="http://www.sciencemadness.org/scipics/axt/peroxodioxime.jpg"></center>
Maybe the structure above is a little optimistic for H2O2.<br><br>
[Edited on 9-12-2005 by Axt]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Interesting information you got here.
I doubt you'd get the peroxy bond in the DINA so easily, however- as the whole structure isn't so resonance stabilised. Btw... the left
molecule in the top diagram has a COO missing- as it suddenly appears in the right one.
Anyway, you can easily get hydroxylamine salts from photographic suppliers.
[Edited on 8-12-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
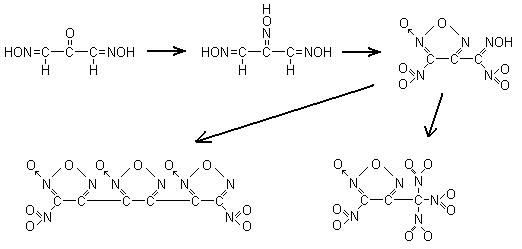
If diisonitrosoacetone was to condense with hydroxylamine to produce trioximinopropane it seems like a nice way to OTC furazans and furoxans. The
article I requested and retrieved by arkansas here: http://www.sciencemadness.org/talk/viewthread.php?action=att...
Shows the reation of N2O4 with trioximinopropape to produce a nitrofuroxannitrolic acid, not so useful itself as it would be too unstable, and while
its salts may be primary explosives, it seems a waste of a good molecule.
Anyways so I looked for possible reactions to convert the nitrolic acid moiety into something more useful. A few examples were found such that by
analogy would yield dinitrotrifuroxan or convert the nitrolic acid into a trinitromethane moiety, which should be exceptionally dense and powerful
explosives. Kinda neat if you could convert citric acid and nitromethane into these 
[Edited on 2-4-2007 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
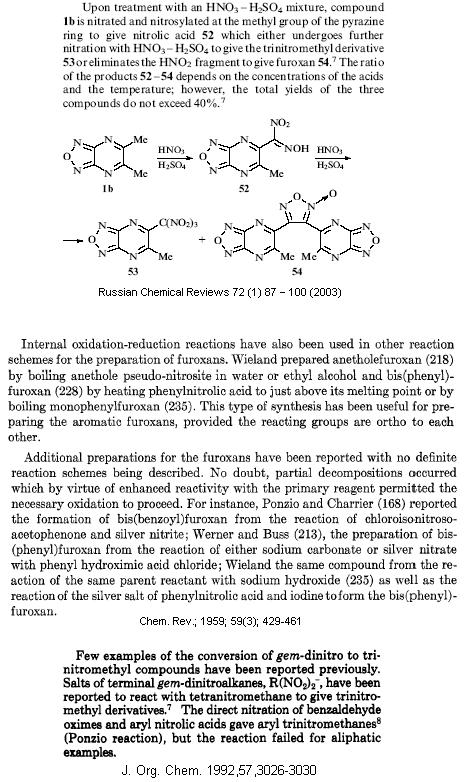
Heres the extracts showing examples of aromatic nitrolic acids to furoxan and trinitromethanes.
[Edited on 2-4-2007 by Axt]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
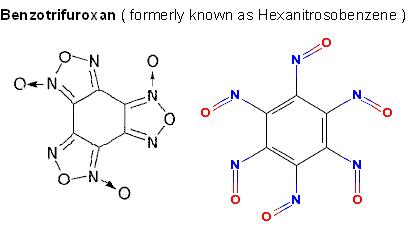
The pathways are only limited by the imagination.
Page 437 of COPAE describes how Trinitrotriazidobenzene decomposes into
Hexanitrosobenzene which is the tautormer Benzotrifuroxan , comparable to
Tetryl in performance but thats nothing to get excited over.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Benzotrifuroxan has been well studied, and far exceeds tetryl in performance. Its performance is slightly better then RDX though a little bit more
sensitive and far more expensive.
Detonation pressures kbar @ g/cm3
Tetryl = 260 @1.71
PETN = 335 @ 1.77
RDX = 338 @1.767
BTF = 360 @ 1.859
Figures taken from LLNL handbook.
A linear dinitrotrifuroxan could well be more dense then BTF (which is a bit over 1.9g/cm3), better OB, but will most probably be more reactive and
sensitive. The nitrofuroxan moiety is a very dense one, dinitroazofuroxan O2N(Fx)-N=N-(Fx)NO2 has a density of ~2g/cm3 and VOD around 10000m/s.
[Edited on 3-4-2007 by Axt]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
As an area for research this seems odd to me. On one hand endeavoring to obtain
a stable unreactive material which meets or exceeds stringent military requirments
and seeking to identify said substance in a class of highly strained structures
which must inherently be unstable and tend to degrade, duh.
http://www.sciencemadness.org/talk/viewthread.php?tid=1177#p...
Cubane is only exceptional because it is a platonic form, and these uncommonly
resist change. Tetranitromethane blended with furoxan compounds compliments
and enhances their performance, so even in that these can be improved upon,
so they may serve well as a component with other explosives but unlikely to
overcome characteristic shortcomings.
.
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
As an area for research this seems odd to me. On one hand endeavoring to obtain
a stable unreactive material which meets or exceeds stringent military requirments
and seeking to identify said substance in a class of highly strained structures
which must inherently be unstable and tend to degrade, duh.
Cubane is only exceptional because it is a platonic form, and these uncommonly
resist change.
. |
Furazan and furoxan rings are stable itself, some of their derivatives are very insensitive, others - have very promising energetic characteristics
and density, and brisance, exceeding HMX (dinitrotrifuroxan ~ 105%). So, there is the reason to seek...
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I'm attaching the two references given in Org Synth, The first from J. Chem. soc is quicker then the one in org syth but with slightly lower yields
(80% compared to 85-90%).
Attachment: Acetonedicarboxylic acid - J. Chem. Soc. 121, 1642 (1922).pdf (738kB)
This file has been downloaded 1615 times
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
This one from Berichte der Deutschen chemischen Gesellschaft, Cant read it but supposedly holds the sythesis of acetonedicarboxylic acid using
concentrated H2SO4 rather then fuming.
Attachment: Acetonedicarboxylic Acid - Ber. 17, 2543 (1884).pdf (82kB)
This file has been downloaded 1149 times
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Translation of article
On the matter of acetonedicarboxylic acid, the reference in Axt's last post says on the synthesis:
If one warms dried citric acid on a waterbath with conc H2SO4, until vigorous foaming (CO2) and H2CO3 occurs (kohlensaure), the cooling liquid
produces upon mixing with water colourless needles of ADA. More can be extracted from the mother liquor with ether. [...] Heating the ADA until just
under its MP (~130 deg C) causes decompostion to acetone and CO2. ADA reacts with phenylhydrazine, and forms with FeCl3 a violet complex.
Wow, and at the end the author states that 'through this communication, the author would like to retain all rights to further working on ADA' - if
people did that these days, how much different science would be!
Anyway, this seems like a nice easy prep for acetone dicarboxylic acid.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
I have a JACS article written by a physical chemist investigating the reaction with various amounts of water and other variables, so here it is. The
only product isolated is CO, but even so it sheds a little light on the subject.
Attachment: 52_4729_1930.pdf (566kB)
This file has been downloaded 2223 times
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Interesting, pity it doesn't provide any details in the ber. article. And about the reaction rate, mine took hours to finish bubbling when I did it in
concentrated H2SO4 while another article I found J. Am. Chem. Soc.; 1918; 40(11); 1716-1726 takes ~55min with fuming H2SO4 though they did run it at a
higher temperature they used far larger quantities.
Anyway, I requested a couple more articles which were retrieved by vovan78, first is a later article by the same authors as the nitrofuroxane nitrolic
acid article above. It shows that the same reaction with N2O4 at higher temperatures results in 4-nitro-3-cyanofuroxan C3N4O4. Which seems to be
stable, should be a reasonably powerful explosive and melts at 48-49° thus castable. It also notes that the nitrolic acid itself is relatively stable
thus its salts with ammonia and hydrazine would still retain very good oxygen balance.
http://www.sciencemadness.org/talk/viewthread.php?action=att...
The second was retrieved to give an example of nitrolic acid reaction with HN3 to yield tetrazole derivatives.
http://www.sciencemadness.org/talk/viewthread.php?action=att...
I've looked for information on trioximinopropane but cant find any references to its synthesis or properties, only have a CAS #555-72-6. Good if
someone could check out CAS for more info.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There is a whole different use for DINA, because it is a precursor for carbonyl cyanide C(=O)(CN)2 or in other words, malononitrile with a carbonyl in
the 2 position.
Ths is a liquid, very sensitive to moisture in the air and thus best handled in a good drybox or inert atmosphere. The slightest moisture decomposes
it violently to HCN and CO2
C3N2O + H2O -> 2 HCN + CO2
Sartori says it is prepared from DINA.
Org.Syn.'s prep is from malononitrile in three steps.
As for DINA, we have elaborated elsewhere on the forum quite a few more efficient preps of acetonedicarboxylic acid. Indeed it is worthwhile to dry
the citric acid, and to employ the strongest H2SO4 possible. Oleum is desirable but not mandatory. The acid cannot be stored for long unless traces of
sulfuric acid are very thoroughly removed by washing. Or else it falls apart to acetone and CO2.
I will reinvestigate the prep from DINA of the carbonyl cyanide and compare to the Org.Syn. method.
Here's the reference to the pyrolysis of the diacetyl derivative of DINA to carbonyl cyanide
R. Malachowski, L. Jurkiewicz, and J. Wojtowicz, Ber. Dtsch. Chem. Ges., 70, 1012 (1937).
which according to Org.Syn. suffers from low yield, nonreproducibility and risk of explosion.
The nonreproducibility part is fairly common with pyrolysis procedures, since the geometry of the solid in relation to the heating, the scale, the
rate of heating, and so on all have their roles.
The Org.Syn procedure which is spread over 3 articles, was based on the following:
W. J. Linn, O. W. Webster, and R. E. Benson, J. Am. Chem. Soc., 87, 3651 (1965); W. J. Linn, U.S. Pat. 3,115,517 (1963)
See the Cyanoformyl Chloride and Carbonyl Cyanide thread for elaboration on the carbonyl cyanide.
[Edited on 26-5-2007 by Sauron]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I have the Malachowski article, but yeh wasn't as interesting as hoped due to the issues the orgsyn prep mentions. Probably more interesting to
convert it directly to sodium cyanide, its mentioned <a
href="http://0-www.jstor.org.raulib.rau.ac.za/view/09501207/ap000451/00a00060/0?currentResult=09501207%2bap000451%2b00a00060%2b0%2cFF1D&searchUrl=
http%3A%2F%2Fwww.jstor.org%2Fsearch%2FBasicResults%3Fhp%3D25%26si%3D1%26gw%3Djtx%26jtxsi%3D1%26jcpsi%3D1%26artsi%3D1%26Query%3DTriaminopropane%2Band%2
BIts%2BComplex%2BMetallic%2BCompounds%26wc%3Don">here</a> that "Since the crude acetonedicarboxylic acid contains sulphuric acid, and the
diisonitrosoacetone is contaminated with sodium cyanide...." I've also seen it mentioned as a "cyanide producer" so it is formed in the course of the
reaction or on its decomposition, just dont know how, or how to adapt it.
I was hoping it would just be the decomposition product of Na-DINA by boiling an aqueous solution.
[Edited on 31-5-2007 by Axt]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Apparently HCN is a mjor product of the detonation or deflagration of DINA.
Despite the name von Pechmann gave to it, DINA is an oxime, a di-oxime, and would be formed equally well by treating mesoxandehyde (or
ketomalonaldehyde or more properly 1,2,3-propanetrione) with hydroxylamine - 2 equivalents. I have not yet figured out how to protect the 2 position,
I am working on how to produce the aldehyde.
And that turns out to be by degradation of D-glucose with NaOH in presence of lead acetate. Whether or not this is easier/cheaper than
dehydrating/decarboxylating citric acid with sulfuric acid, remains to be seen.
With DINA in hand by either method I believe it can be made to undergoe a double Beckmann rearrangement by which an aldoxime is converted to a
nitrile. Maybe this needs to be done steprise rather than simultaneously to both oximino moieties. There are a number of classical Beckmann reagents
but the latest is TCT/DMF adduct in DMF.
DINA and MINA the monoisonitrosoacetone are both of current interest as you know not only as energetics and precursors of energetics but also as
colorimetric reagents for OPAs and as reactivators of human ACEase that has been inhibited by an OPA. For that purpose the cyanogenicity is not a
positive factor. My own interest is less with technology than chemistry.
I changed the name of the Carbonyl Cyanide etc thread to Glucose ->DINA -> Derivs so pls have a look. Please post in there as I'd like to be
able to reply to someone other than myself, and it seems you and I are the only ones interested.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I've been searching for a literature synthesis of trioximinopropane (aka tris(hydroximino)propane, trinitrosopropane, trioximinoacetone etc.), though
always hit a dead end.
The following is the best I have found regarding its synthesis, found in Proceedings of the Royal Society of London. Series A, Vol. 107, No. 741.
(Jan. 1, 1925), pp. 80-92.
"A different synthesis was then attempted. Citric acid was converted into acetonedicarboxylic acid by Jerdan's modification (' J. Chem. Soc.,' vol.
75, p. 809 (1899) ) of von Pechmann's method ((Ann.,' vol. 261, p. 155 (1891) ). The crude dicarboxylic acid on treatment with sodium nitrite solution
(von Pechmann and TVehsarg, 'Ber.,' vol. 19, . 2465 (1886)) gave diisonitrosoacetone, which was in turn converted to trioximinoacetone (' Ber.,' vol.
21, p. 299 (1888) ). All attempts to reduce trioximinoacetone to triaminopropane failed, in spite of the use of many reducing agents under very varied
conditions ; in each case the formation of ammonia indicated disruption of the trioximinoacetone complex. The diisonitrosoacetone was therefore
reduced with stannous chloride (Kalischer, ' Ber.,' vol. 28, p. 1520 (1895) ), and diaminoacetone isolated as its dihydrochloride."
So yes it is derived from diisonitrosoacetone, presumably with NH2OH though I've never seen it stated as such. I requested the <a
href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=9319&pid=109981">Ber article</a> which it cites, though
I see nothing of relevance in it. Maybe a german speaker can have a look over it and try identify anything interesting.
As previously shown i this thread, trioximinopropane when reacted with NO2 yields nitrocyanofuroxan (C3N4O4, MP 48-49°C). NCF's calculated explosive
properties are given in Journal of Molecular Structure: THEOCHEM 765 (2006) 77–83.
Hf = +333.59 kJ/mol
Density = 1.8 g/cm3
Heat of detonation = 6.076 kJ/g
VOD = 8970 m/s
Det. Pressure = 35.72 GPa
Thus its calculated properties are simular to RDX.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Trioximinopropane is the tri-oxime of mesoxaldehyde, the simplest possible tricarbonyl compound. DINA is one of the routes to this.
The others are hydroxypyruvaldehyde which is usually stored as a cyclic trimer, or dihydroxyacetone usually stored as its cyclic dimer (commercially
available).
And both of these can be made from carbohydrates or glycerol or acrolein and so on.
I seem to recall a preparation of the trihydrazone from one or the other dihydrazones. The hydrazone is readily convertible to the oxime by treatment
with hydroxylamine.
This was in JACS and it referenced an old Pechmann article from Ber.
Jerdan's method for acetonedicarboxylic acid is rather passe as it was found to be unreliable or at least, highly variable in yield. Alternatives have
been found that are just as convenient and more consistent, I believe I already advised you in PM about this.
[Edited on 15-11-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
"which was in turn converted to trioximinoacetone (' Ber.,' vol. 21, p. 299 (1888) )."
I requested the <a href="http://www.sciencemadness.org/talk/viewthread.php?action=attachment&tid=9319&pid=109981">Ber article</a>
which it cites, though I see nothing of relevance in it.
|
The reason being it was an error, this was found;
"Ni(OAc)2 added to alc. H(C:NOH)3H (cf. Ber. 21, 2991(1888)) ppts. the complex Ni salt (C3H4O3N3)2Ni, orange-red, decomps. without fusion around
280°, also formed by heating aq. H(C:NOH)H with metallic Ni. Aq. HC(:NOH)C(:NOH)H and metallic Ni heated on a water bath form immediately a
colloidal soln. of the complex Ni salt (C2H3O2N2)Ni, but on continued heating a brown-yellow ppt."
So the correct reference needed is:
H. v. Pechmann, K. Wehsarg "Ueber Dinitrosoaceton" Berichte der deutschen chemischen Gesellschaft, Volume 21, Issue 2, 2989-2993 (1888).
http://dx.doi.org/10.1002/cber.188802102148
I dont think I have that one, so if anyone reading this with access to it, attaching it into this thread will be appreciated.
|
|
|
| Pages:
1
2 |