| Pages:
1
2
3
..
15 |
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Mystery Glassware Identification Thread
I recently received a rather large treasure trove of glassware from a friend who is a chem professor. Incredibly, he found all of it in or by the
dumpster when he was leaving work!!!
This piece is stuck to the top of a Vigreux column. My friend had no idea what it was other than the obvious: it was part of a distillation setup. I
have searched google, and posted it on another forum. Someone finally suggested that it is probably a distillation splash head, and I think he's
probably right, but I thought I'd post it here and see what the community here says. After all, as the someone also pointed out, if it is a splash
head it would be pretty pointless at the top of a fractionating column.

[Edited on 1-13-2016 by zts16]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I have no idea what that is, but I must say this is a great thread to have! I was actually going to start one myself, because I too stumbled on a
glassware jackpot the other day and have received plenty of odd contraptions. I'll have pictures up here in a few days!
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
hmmm--if its 24/xx I'd be willing to give it a home and let ya know.......................LOL
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
That could be a reactor of some sort: you distill something and you put a gas inlter so the gas can react in the bulb, make a higher boiling point
compound and return to the reaction vessel. But really, I'm just devagating here.
It could also be just the top of a fancy reflux setup that would take a drying tube in the top of the bulb. Or without the drying tube.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Maybe it's a splash head for Kjeldahl distillation, albeit a somewhat unconventional one? There's a lot of custom glass out there that glassblowers
put together on request.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
prof_genius
Hazard to Others
  
Posts: 147
Registered: 15-5-2013
Member Is Offline
Mood: No Mood
|
|
It looks like custom glass, I recommend looking through old glassware catalogs, they tend to have this type of interesting and unusual glassware.
Maybe ask a glassblower or someone at the university it was found.
|
|
|
Ascaridole
Hazard to Self
 
Posts: 67
Registered: 11-9-2013
Location: Hawaii, USA
Member Is Offline
Mood: Searching for glass....
|
|
I'd second UC's idea of a Kjeldahl trap. Kinda interesting design actually, vapor can enter in the top and distillate can return on the bottom.
Oh, just notice the spring barbs... adds to the mystery...
[Edited on 16-7-2014 by Ascaridole]
Ascaridole, the masked bandit of chemistry!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Any idea what this is? Basically it's a hollow 24/40 stopper with a little hook in it. My best guess is that it's for hanging something from inside
the reaction vessel, but I have no idea what, or if that's actually what it is.
There will be plenty more mystery glassware pics as I continue to unpack more of it!

|
|
|
Ascaridole
Hazard to Self
 
Posts: 67
Registered: 11-9-2013
Location: Hawaii, USA
Member Is Offline
Mood: Searching for glass....
|
|
Thermometer hanger for full immersion thermometers so you can measure the vapor temperature at the head of a still or above a reaction accurately
(like in semi commercial prep, not really for regular <1L size stuff). If you use a standard thermometer adapter with a partial immersion
thermometer you need to calibrate it to account for the under/over immersion in the vapor stream but if you use a full immersion, yay no calibration
needed.
It is also used to hang catalytic material in vapor phase reactions such as platinized mesh or copper mesh.
I think there was one more use for it but it escapes my mind at the moment....
Ascaridole, the masked bandit of chemistry!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Aha! That makes sense, thank you.
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
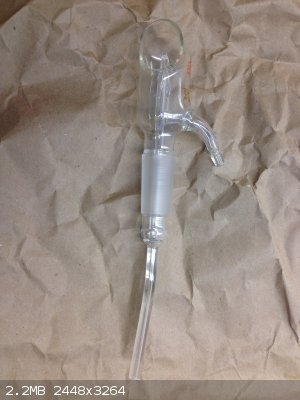
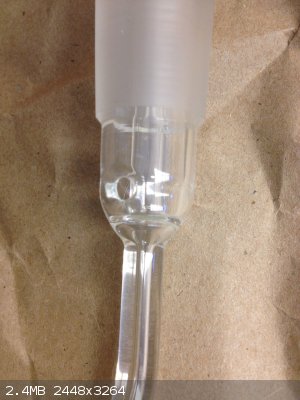
Here's another one. So bizzare because I can't think of anyway to cover the open face, which I'm guessing will have liquid or gas spewing out from
the inner spout.
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
That's a TLC sprayer, bottom suction tube is place into a staining solution and then air is passed through the side-arm. This will suck staining
solution to the steam of air (thanks to venturi effect) and gives a uniform spray coating on a TLC plate.
[Edited on 18-7-2014 by kavu]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Alright, I've got two things here:
 
Neither belong to me, but they came from the same place that I got a lot of my equipment from.
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kavu  | That's a TLC sprayer, bottom suction tube is place into a staining solution and then air is passed through the side-arm. This will suck staining
solution to the steam of air (thanks to venturi effect) and gives a uniform spray coating on a TLC plate.
[Edited on 18-7-2014 by kavu] |
Thank You! It's great to start putting functions to faces. Sounds like it will go great with the TLC plates, and such that were also in the trove.
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Mystery Glassware II
Here's another piece that I could use help identifying:

|
|
|
Ascaridole
Hazard to Self
 
Posts: 67
Registered: 11-9-2013
Location: Hawaii, USA
Member Is Offline
Mood: Searching for glass....
|
|
Wow that is an unusual setup definitely custom. Its used to dry material very sensitive to thermal decomposition.
Solvent vapor enters on the top (of photo) and fill the jacket. A ST joint 10/30? (bottom of photo) thermometer goes into the bottom and is secured
using the spring barbs. The vapor exits through the right side (of photo). The sample is loaded through the male ST joint on the top and connected to
a vapor trap or another flask to condense the vapors coming off the sample.
Usually this is used to dry samples that are very easily decomposed under elevated temperatures. In your case this apparatus is special. Its design
allows it to be used under mild pressure. This method of drying is very very slow but you don't get hotspots and charring of material as the vapor
helps to keep even heating.
Also yes why the new thread?
Ascaridole, the masked bandit of chemistry!
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ascaridole  | Wow that is an unusual setup definitely custom. Its used to dry material very sensitive to thermal decomposition.
Solvent vapor enters on the top (of photo) and fill the jacket. A ST joint 10/30? (bottom of photo) thermometer goes into the bottom and is secured
using the spring barbs. The vapor exits through the right side (of photo). The sample is loaded through the male ST joint on the top and connected to
a vapor trap or another flask to condense the vapors coming off the sample.
Usually this is used to dry samples that are very easily decomposed under elevated temperatures. In your case this apparatus is special. Its design
allows it to be used under mild pressure. This method of drying is very very slow but you don't get hotspots and charring of material as the vapor
helps to keep even heating.
Also yes why the new thread? |
Wow, thank you for the explanation. I don't know when I'll have a chance to use it but it's good to know what it is.
|
|
|
electrokinetic
Hazard to Self
 
Posts: 52
Registered: 6-6-2012
Location: Rhuidean
Member Is Offline
Mood: No Mood
|
|
Let's call this #6. It's an Ehrlenmeyer flask with a tube running through it. The picture doesn't show it but the tube is open at both ends. My
guess is it's custom.

[Edited on 20-7-2014 by electrokinetic]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Alright, well here's #7 then (Which would make the two in my last post #4 and #5 from left to right)
It looks like a drying tube to me, but it's closed on one end, and looks rather unusual, so I wasn't sure.

[Edited on 7-19-2014 by zts16]
|
|
|
prof_genius
Hazard to Others
  
Posts: 147
Registered: 15-5-2013
Member Is Offline
Mood: No Mood
|
|
#6 is probably used to dissolve a gas in a liquid.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah, I think you're probably right about that. It would
be quite a useful piece to have.
|
|
|
prof_genius
Hazard to Others
  
Posts: 147
Registered: 15-5-2013
Member Is Offline
Mood: No Mood
|
|
I'm thinking of finding a local glassblower and having them make me one.
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
I believe that the "drying tube" is for fermentation, IIRC. Filling it with a bacterial culture and turning it upright will collect any gas that is
produced.
Fear is what you get when caution wasn't enough.
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
I cannot recall the exact use of #7 but as far as I remember it's used in biochem to measure gas formation from samples. The tube is filled with water
and the sample is inserted to the bent part. As gas is formed it is collected to the closed end of the tube.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I take it that the spring barbs are the little
pointy things on there near the joints? What are they for? I have several pieces that have them and I was rather mystified.
|
|
|
| Pages:
1
2
3
..
15 |