charles
Harmless

Posts: 10
Registered: 10-2-2005
Member Is Offline
Mood: No Mood
|
|
eugenol demethylation via MICROWAVES
hi,
i'm new here so be nice with me  
some time ago i found this procedure on some archive, where someone demethylated eugenol: the single Met-O- group to get -OH
here's what he wrote:
| Quote: |
Eugenol Demethylation with Pyridine HCl (by Startinout) [4]
Basically the microwave demethylation fucking rocks, as far as ease goes, but the downside is because you need 5:1 pyridine HCl:eugenol.
I made my pyridine HCl by gassing pyridine with anhydrous HCl gas, this was pretty easy (just gassed it for a while, some crystals formed but it was
quite hot so i froze it, many more crystals formed, filtered it, washed with ether, then put the crystals on the bench to dry, came back, almost no
crystals left and very sloppy, note-they are very deliquescent and they sublime so put them in a closed container immediately post production.
Microwave Demethylation
All I did was added eugenol in the weight ratio five parts pyridine HCl two parts eugenol (this is molar 5:1), put this in a round bottom flask,
stoppered it, microwaved in a normal microwave on medium low (the paper uses 215 watt oven) for two minutes, the stuff melts and turns quite hot, it
was removed, cooled under the tap until it was room temp again (just felt it with my hand), then microwaved again for two minutes etc etc, this
process was repeated six times, the more you do it the hotter it gets each time. After the sixth time i cooled it again, poured some cold water from
the fridge into into it and extracted it three times with ether, pooled the ether extracts, removed the ether on the rotavap, the residue had no smell
of clove oil whatsoever, just the smokey smell of the demethylated product, so i assumed quantitative yeild cause I couldn't be fucked vac
distilling a room temp solid and/or GC'ing it, also the methylation requires an excess of all other reagants anyway.
|
SO, what do you think about?
it's really so easy the demethylation of a methoxy group on eugenol using microwaves and some pyridineHCL xtls?
isn't dangerous to put liquid pyridine in a microwave owen?
tnx for helps 
|
|
|
enima
Hazard to Self
 
Posts: 98
Registered: 1-12-2004
Member Is Offline
Mood: No Mood
|
|
Sounds expensive. Your probably better demethylating the eugenol with SIBX
You need 5 molar excess of pyridine. Pyridine aint cheap. Whats your goal?
safrole?
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Yes the end result would be a compound which you can make safrole from....
That synth was on rhodiums archive, a review of the demthylation process, if you can get pyridine, catagory 1 (highest) drug precursor restricted
chemical in australia (dont know about other countries) your doing well, the 5:1 ratio is bad as pyridine, if you can get it, is not cheap....
And its not just whack them together in a bowl nuke it and take it out.... you need to have calculated exposure otherwise you may harm the substrate.
On the issue of eugenol demthylation the only route i have seen which is viable is the use of AlI3 and PTC under inert atmosphere reflux. There was
quite a lengthy disscusion back at the hive about PTC's for this proceedure... Dont remember where it lead to tho.... think it was headed in the
direction of PEG's substituted for TBAI as in the proceedure... cant remember sorry....
Or there is the SIBX route as mentioned, either way, but steer away from pyridine, its far to risky to try and buy it....
-rlr
|
|
|
charles
Harmless

Posts: 10
Registered: 10-2-2005
Member Is Offline
Mood: No Mood
|
|
yes the goal is something that can be "metilenated" -O-CH2-O-
here pyridine isn't so watched or in tabel...
and it's expensive but not so much... like buying sassy root to stream distill to get a 7% by weigh oil. 
so... it is not a viable route, tnx for the help 
can you tell me something about AlCl3 and PTC (phase transfer catalyst?) in inert atmosphere? i dunno if it's easy work under intert
atmosphere... i have a proceudure to get AlCl3 by Al powder, but it really need to be under N2 or other gas....  
** what is SIBX?
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
| Quote: | Originally posted by runlabrun
And its not just whack them together in a bowl nuke it and take it out.... you need to have calculated exposure otherwise you may harm the substrate.
|
Yeah well not only the substrate, if your reproductive system isn't included I mean  (This affects mostly man, the majority around here I assume). Be careful. I can buy pyridine but the procedure seems too expensive when
compared to other demethylations I have heard/read about. (This affects mostly man, the majority around here I assume). Be careful. I can buy pyridine but the procedure seems too expensive when
compared to other demethylations I have heard/read about.
Theory guides, experiment decides.
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Haha, yeah there is always that factor about pyridine.... try to steer away or lose your ability to "sow the seed"....
PTC = Phase transfer catalyst
The method of refluxing eugenol, TBAI (tetrabutylammonium iodide this is the PTC) and AlI3 under inert atmosphere, argon is easy OTC inert atmosphere
from a welding store. For this method the inert atmosphere is a must.
SIBX is stabilised 2-iodoxybenzoic acid.
Check this out for info, has direct info on its use for what your looking at... handy....
http://www.simafex.com/_pdf/organic_letters.pdf
-rlr
EDIT: I really must learn to spell.....
[Edited on 12-2-2005 by runlabrun]
|
|
|
daeron
Hazard to Self
 
Posts: 74
Registered: 26-3-2005
Member Is Offline
Mood: cancerogenic
|
|
Hahaha I cant believe it someone is actually interested!!!
Yes swim done it a couple of times and it works perfectly if swiy has a heat sink by. Used a MW w 2.5cm diameter holes connected to the hoover(fume
hood). Real Hazmat gloves used, w gasmask as well. If swiy smart nothing wrong will happen.Did swim mention that it works perfectly…PERFECTLY!!!
Swim tweaked the ratios a bit, and wrote it down but he was forced to “leave in a hurry” so he doesn’t have the notes now…he sent some of them
to Joe Aldehyde and some others so maybe they will be so kind to repost them,hmmm?
If there are Qs ask away!
Btw the continuation(never got around to complete it in a full MW fashon): http://www.sciencemadness.org/talk/viewthread.php?tid=3648
|
|
|
againa
Harmless

Posts: 2
Registered: 28-4-2005
Member Is Offline
Mood: No Mood
|
|
Kiss and tell
Daeron,
 Believe it! Believe it!
Very interested please share All the details.
Did you make your own pyridine-hcl?
Your work up?
Recycle the pyridine?
Time?
Scale?
Yeild?
Wattage micro?
Any one care to muse on bubbling the fumes through solution of NH4OH?
How, How, details, juicy details, sweet, luscious, complete overkill, too much information, details!!!
Sorry, there really aren’t too many specific questions….cuz I want to know everything!
Joe!!!!! Help him out!!!!
Thank you so much for your time
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
Review of aryl-methyl ether cleavage.
The aryl-methyl group present in Eugenol has long be known as one of the most difficult ether groups to cleave. The earliest records for the
demethylation of this group use a HI acid reflux. This won't work with Eugenol because HI will first add across the alkene. Methods using AlCl3
can't really be recommended either. The use of pyridine dates from the 1930s. Lewis acids such as AlCl3 have also been used. Recent promising
results have mostly used microwave heating using AlI3, Pyridine HCl, SIBX or CH3SO3H.
1) Using Aluminium Iodide. This method is messy. [ref. 6]
2) Pyridine hydrochloride. There are many reports of Pyridine (with and without Aluminium Chloride) being used to cleave aryl-methyl ethers. Earlier
reports required autoclaves. This method, using microwaves, was tested by Startinout at the hive and found to be quite easy. People have slated it
because Pyridine is itself said to cause male infertility and smells absolutely horrid. The published method needed 5 moles of pyridine per mole of
aryl-methyl (i.e. Eugenol). [Refs. 1, 2]
3) Using SIBX. [ref. 5]
4) Using methanesulfonic acid (CH3SO3H). This has been recently demonstrated (2002) to work for anisole but not for Eugenol, but there is no evidence
that the researchers actually tried Eugenol.[ref. 3]. The method is promising because strong acids (HI, HBr) have been used in the past for
aryl-methyl demethylations and also weak acids such as ordinary carboxylics and their salts (see USP 4473713). More recently carboxylic acids have
been used with microwave heating to cleave aryl-methyl ethers. [ref. 4]
I haven't made any attempt to discuss the older or trickier methods. Only the 4 methods above look worth pursuing. However I have included
examples in the documents I have uploaded here:
http://rapidshare.de/files/6213173/Demythylations.zip.html
The documents in this archive include some duplication and repetition. Has anyone else noticed how long it takes to upload small documents to
"rapidshare". Presumably the longer the upload takes the more time one spends looking at their adverts.
References.
1. "Piperonal and Safrole from Vanillin and Eugenol.html", Rhodium archive.
2. Kulkarni, Kadam, Mane, Uday, Desai, Wadgaonkar, "Demethylation of Methyl Aryl Ethers using Pyridine Hydrochloride in Solvent-free Conditions
under Microwave Irradiation.", J. Chem. Research (S), 1999, pp 394-395.
3. Anna Fredriksson and Sharon Stone-Elander. "Rapid microwave-assisted cleavage of methyl phenyl ethers: new method for synthesizing desmethyl
precursors and for removing protecting groups". Journal of Labelled Compounds and Radiopharmaceuticals (J. Label. Compd. Radiopharm. 2002; 45:
529-538).
4. G Majetich & R Hicks. "Cleavage of aryl ethers with microwave heating" page 578 of Radiat. Phys. Chem. 45(4), 567-79. (e.g.
4-MeO-acetophenone to 4-HO-acetophenone). See document "ArOMe_Demythylation.htm".
5. Ozanne, Pouységu, Depernet, François, Quideau. "A Stabilized Formulation of IBX (SIBX) for Safe Oxidation Reactions Including a New
Oxidative Demethylation of Phenolic Methyl Aryl Ethers". Organic Letters, 2003, Vol. 5, No. 16, p2903-6
6. (i) Synthesis, April 1985, pp 437-9. (ii) USP 4695659. (iii) See document "ArOMe_Demythylation.htm" for experimental procedure.
The following US patents describe some interesting demethylations:
4967013 Preparation of hydroxybenzaldehydes
4667037 Dealkylation of opioid ethers
5071985 Process for the preparation of morphinane derivatives
4695659 Method for dealkylation of alkyl-aryl ethers
5648552 Process for the preparation of isovanillin
To get these go to:
http://www.freepatentsonline.com/search.html
Enter, for instance US4967013 into the 'Patent Number' box and Search.
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
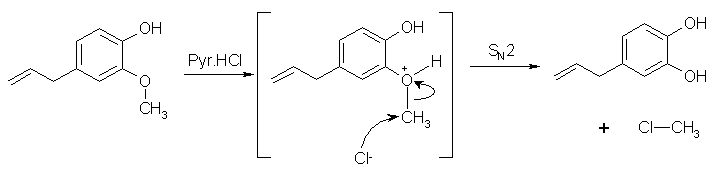
Startinout's pyridine demethylation
I believe this is SN2 acid demethylation. [pyridine.HCl is the "acid" here; the salt of a weak base and a strong acid]
eugenol + pyridine.HCl ==> MeCl + allyl-catechol + pyridine.
MeCl is a "potential carcinogen" so you should put your microwave in a fume cupboard and evacuate the fumes outdoors. [MeCl has a Merck entry, under
methyl chloride]
During the Startinout's workup you need to remember that an ether extract will contain pyridine as well as allyl-catechol. They can be separated using
A/B methods. I.e wash with dil aq. acid x times to remove pyridine or wash with dil. aq. base x times to extract allyl catechol.
Startinout's procedure is here:
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/m...
|
|
|
|