| Pages:
1
2
3 |
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Bismuth Crystals, etc.
I thought I might share my tinkerings with this beautiful element.
Bi forms hopper crystals, which I’m sure we’ve all seen at museum gift shops at one time or another without realizing what they are. Here is my
(improvised) procedure on growing these things. I hope that someone here finds it useful.
First, get some bismuth. Get the highest purity you can. 99.99% is a good, cheap grade available on ebay, here, here, and many other places.
Next, get a small crucible to melt your bismuth. I have used a 10 mL beaker and the snuffer cap of my alcohol burner successfully for this. A word
about metals: if you use a metal crucible, make sure that you melt your bismuth in the presence of air. This creates an oxide coating which separates
the bismuth from the crucible and thus prevents alloying.
Melt enough bismuth in your crucible to produce a roughly cylindrical melt and remove the heat source shortly after the charge finishes melting. Do
the heating with a small heat source; I’ve found that a simple alcohol burner works perfectly for both the cap and the beaker. The trick is to get
all of the bismuth molten, but at as low a temperature as possible. If there is any solid bismuth around the sides, crystals will grow inward instead
of outward. If the temperature is too high, proper nucleation will be difficult, but not impossible.
At this point you have a melt of liquid bismuth that is ready to crystallize around any cool object it touches. Place the points of a pair of pointed
tweezers 1-2 mm into the surface of the charge and bring the tips together. This will give you two or more seed crystals firmly attached to the
tweezers.
At this point, you should quickly skim the layer of gray bismuth oxide off the top of the melt. A new layer will immediately start forming, but it
will be highly colored and give the top of your crystals a very nice coloration.
While waiting for the crystal to grow (this usually takes around 20 seconds), make sure to slowly and gently shake the tweezers around to get a feel
for the size of the crystal. When it has reached the edges of the crucible, pull out the tweezers with the crystal mass on them and shake the whole
thing violently until no more liquid bismuth flies off. Do this over a sheet of aluminum foil so the bulk of the bismuth gobs can be recovered. Make
sure to insulate your hand, as molten bismuth will give second-degree burns easily. You can wear a glove, but I’ve found that most of the molten
gobs land on the middle segment of my middle finger.
Wait for the crystal to cool down and remove it from the tweezers. You now have a colorful crystal mass comprised of several crystals with very sharp
hopper formations (exposed by the shaking) and two small holes where the tweezer tips were. I will upload some pictures as soon as I can figure out
how to take decent pictures of the crystals with my camera.
A word about the pesky oxide: you will get a lot of useless oxide mixed with metallic bismuth from the skimming. To recover the metal, simply heat the
stuff in a test tube to about red heat. The oxide will melt and separate from the metallic bismuth, which can now simply be poured out and remelted.
Another form of bismuth that is a sight to behold is plates. If grown correctly, they can be very beautiful and sometimes even include patches of
brilliant color. Plates are best grown under an inert liquid—cheap cooking oil works, but be careful not to overheat it or it will smoke and release
a mild lachrymator.
The first thing to do when growing a plate is to remove all of the oxide on the bismuth. This can easily be done by placing bismuth in oil and
stirring it rapidly. The oxide will come off as a black precipitate and leave clean, shiny bismuth at the bottom.
Melt your clean bismuth under clean oil in a beaker. Remove the beaker from your heat source and put it on a thin sheet of metal. What sheet to use
varies, but I’ve found that my aluminum sheet kitchen sink works. If you get the correct rate of cooling, you will get a very nice plate with
clearly visible crystal structures. If it cools too quickly, you will get a plate with a scaly top. Too slow and you get a plate composed of a few
large crystals with few boundaries.
A variation on this method is to melt the bismuth in the cover plate of a glass Petri dish, covered by the bottom plate (under oil, of course). When
the bismuth melts, turn off the heat source and put some weight on the dish. If all goes well, you will get a large, thin plate of bismuth. The rate
of cooling is not as important with this method, but be careful not to overheat, as the low heat capacity of the whole system makes this all too easy.
Also make sure to add the proper amount of oil, a very tricky feat with little room for error.
Occasionally, you will get a patch of intense color on one of the crystal faces. I can’t fully explain this, but my best guess is that this happens
when a crystal grows at just the right angle with the glass surface and creates something like a diffraction grating.
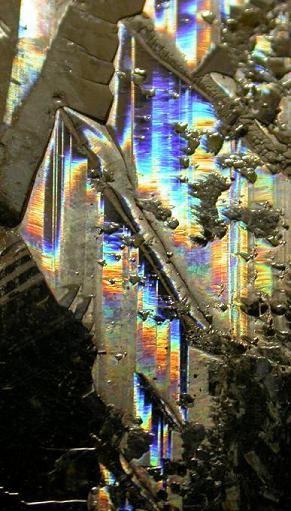
Finally, here is a cropped image of a part of the bottom of a plate grown in a beaker.

|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Here is a picture of one of these spots. Oddly, this batch of bismuth is the only one I got these colors with. The new, purer batch I melted down
produced far fewer patches and all much less colored. I can’t tell you what was in this batch, as I’d remelted the stuff so many times I don’t
know what could be in it.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Great stuff, thanks for sharing.
I wonder what factors influence crystal shape. For instance I have seen Bi crystals (not with my own eyes though) that were very regular, still
somewhat jagged.
I wonder what factors could be applied to achieve smooth crystal growth - i.e. crystals that grow several cm in diameter, and yet show only a handful
of different crystal faces (if that's the term). Obvioulsy, the more jaggy it is, the more it implies either impurities or hesitant/disrupted
irregular crystal growth. Optimal crystal growth normally requires constant and unwavering conditions ( I know that from protein crystallography, so I
reckon this applies here too), hence I suspect that 1) temperature control, 2) a spacially fixed crystallisation nucleus 3) selection of optimum
temperature are of utmost importance.
Further, as an idea, rather than using a tweezer and hoping for random crystals, it might be a good idea to allow the first batch to grow, then to
break off crystals of the desired shape, and use *that* as a nucleus for a second round of crystallisation (A process called seeding).
This should produce nicer crystals.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Thanks for sharing that. I can't wait to see the pics of your hopper crystals 
If only I had some Bi... 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I’m still playing around with my camera, hopefully I’ll get some good pics soon.
Most of the crystals I have grown have 2-3 big crystals and a small one here and there. The big ones are always nicely formed—a pyramid with stepped
sides. There was one exception, though, which grew much more ‘hoppered’ than usual. How jagged a crystal is doesn’t affect the number of faces
you will see, they are always parallel.
I have considered seeding, but this would introduce problems of its own. The crystals are always hoppered/stepped, so getting them into the melt past
the somewhat sticky oxide layer would be a real challenge. Another problem is temperature control. If you put the seed in when the melt’s
temperature is too low, the crystal will not grow properly (as I already explained). Too high and the seed melts, as the crystals are rather thin
(~1mm). If the seed is too cool, it will create a nucleation point where it touches the melt. If its too hot, it will simply melt.
Now that you have me thinking about this, I do have one idea that might prove feasible. If you slowly cooled a molten ingot, it would give you large
internal crystals. Take a hammer to this and you will easily get a number of pyramids. If you melt the very outside of a big one and put it into a hot
melt, it should incorporate itself into the melt properly. If you cool the melt quickly enough, some part of the crystal will remain solid and start
to grow when the temperature gets low enough. If only I hadn’t contaminated my ingots with copper… Oh, well I will get some more Bi sooner or
later.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Here are some pictures, sorry about the lighting. The abnormal one is on top, the most average one I could find is on the bottom. The crystals don’t
usually grow at this angle to each other, this one was also somewhat unique. That’s the problem with only making only two or three dozen of
something: they’re all unique and you can’t get a very average sample.
P.S. Sorry about the double post, it's the only way to upload the pics.

|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
AWesome  I like the neat pyramid one. Im envious I like the neat pyramid one. Im envious 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
tantan
Harmless

Posts: 12
Registered: 15-6-2004
Member Is Offline
Mood: No Mood
|
|
Look very much like professionally-prepared Bi crystals, very beautiful indeed!
FWIW, here's a link to an article about growing bismuth crystals:
http://www.popsci.com/popsci/how2/article/0,20967,714559,00....
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
The crystals are very pretty indeed. You can buy a few ounces of Bismuth from the sporting goods store:
http://www.cabelas.com
Do a search inside the site for Bismuth. The fishing weights are $3 for 3 of the 3/4 oz weights which is over 60 grams for $3. Don't buy the
bismuth shot like I did. I paid too much.
Bismuth can also be used in levitation with strong magnets.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
$3 for 3/4 oz of no-purity Bi? There's a guy on ebay selling the 99.99% stuff for $8/lb. Don't you love shopping around?
I found a plate lying around and decided to grow some crystals. Annoyingly, I kept getting nucleations at the edge of the melt instead of the center.
It seems that my glass crucible (10 mL beaker) likes to stick to the Bi for some reason. It sticks a little higher than the meniscus, cools down
there, starts growing crystals… I don’t remember having this problem before.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
$8 a pound sounds better than $3 for 2 1/4 oz. I should get out more ;-) The shipping calculator adds another $11 to the cost, ouch.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I’ve been working on the sticking problem and finally found a solution. Add a small amount of oil and the Bi won’t stick. Don’t add enough to
see, just add enough to encircle the ring where the meniscus joins the crucible.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
It seems that working with my Bi steadily contaminates it. It must be dissolving my tweezers and steadily becoming contaminated that way. Does anyone
know where I’d find some sort of a chart of what metals Bi forms alloys with?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Are you sure it's not low levels of oxidation, similar to what you get when melting tin and lead?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I tell the approximate purity by the color of the crystals. Nice pure ones will be bright purple and green in some places, contaminated ones will be
dark yellow.
Part of the reason I ask is that I’m trying to make a substitute for my tweezers: a rod of some metal with a slightly mushroomed end. The problem is
that I’m not sure what metal to use. Glass doesn’t seem to have the thermal conductivity necessary and Bi pieces are too hard to make in a shape
that can be firmly held with tweezers of tongs.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Heck, Bi alloys with lots of stuff. Especially in the 0.01% range.
Heck, "insoluble" magnesium carbonate is about that soluble in water as I recall.
I don't know of anything you could add to clean it, you'll have to electroplate it out or something.
Tim
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Hg also alloys with many metals, but iron is a notable exception. There has to be some equivalent here.
Would Al work for this? The surface oxide layer should hold well enough, shouldn’t it?
|
|
|
HNO3
Hazard to Others
  
Posts: 211
Registered: 10-11-2004
Location: America
Member Is Offline
Mood: No Mood
|
|
If you have access to pliers, glass rod or tubing, and a propane torch, you can make a spatula. All you have to do is heat teh end of the rod/tubing
to a red /orange color, and then use gravity to get the glass blob 2-3mm away from the main portion of the rod/ tubing, and sqeeze it with the pliers.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I would think any oxide will be more than suitable. Aluminum, titanium and stainless are passivated by oxide layers.
Glass wouldn't be optimum because bismuth tends to oxidize and that makes it sticky (same reason gallium sticks to glass).
Tim
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Sorry, HNO3, glass doesn’t work. If I use glass, the crystals come out malformed. Immediately around the tip is a crust of solid, but not very
crystalline, Bi. Immediately below this are nice, big crystals. The problem is that almost nothing connects the crystals to the crust and they always
separate when you pull them out of the melt.
My best guess is that the Bi around the glass (which is cold when I put it into the supercooled melt) immediately crystallizes, raising the
temperature of the surrounding melt above the mp of Bi. The melt cools a little and crystals then start growing off of the very bottom of the crust
(by convection, cooler Bi would be farther down) and continue because the lower parts of the melt is cool enough to hold the heat released by
crystallization without going above the mp.
I never had this problem with my tweezers because, I guess, they conducted that initial burst of heat away and allowed crystals to grow correctly.
Tim: The oxides aren’t so much of a problem at these low temperatures. Are you sure about SS being passivated by an oxide layer. It doesn’t seem
right to me that something made of Fe (non-passivating), Cr and Ni (also non-passivating, IIRC) would passivate in this way.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Try looking online at jewlers supplies, you can get fused silica rods which may work better and high purity carbon rods for working with molten gold.
You could probably use these rods for nucleation quite well. You can also buy little disposeable crucibles ment to be heated with a torch ment for
holding molten gold and such, these should be great for working with molten bismuth.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ah, chromium is actually passivated. Most metals around that reactivity passivate; zinc does reasonably well. Stainless has 10-20% Cr which comes to
the surface and oxidizes (or sometimes does not, allowing it to rust; in which case citric or hydrofluoric acids must be used to passivate it).
Tim
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I’ve recently been playing with nucleation using small pieces of Bi and found them to work decently well. To make the rods, I pour liquid Bi into
cold water, giving me something like this:

I pick out the big pieces and cut small notches near their ends (this is where I hold them with my tweezers). To nucleate the melt, I melt the other
end of the piece in a small flame and put it into the melt. Keeping the end of the piece right at the surface and resisting the urge to keep pushing
it in because it’s slowly melting (until the melt cools enough to start crystallizing) is important at this step. The rest of this procedure follows
what I wrote above.
I recently got a pound of 99.99% Bi and have been playing with that. It makes around 50mL when molten, but I’m still trying to work out how to get a
good crystal mass from this. I keep getting random nucleations in the melt and around the edges. I’m still playing with the edges, but I’m lost on
what to do with the ones on the inside. I had this same problem with the smaller crystal masses, but the crystals were too small to be much of a
nuisance. Here, though, they are. I won't know how well picking them out works until I can get the edge problem fixed... this could take a while.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
If I didn't know better, I would've thought that last picture was a pile of fish! 
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Update
Well I'm still playing with the Bi. Looking back on my old ‘snuffer cap’ experiments, I don’t remember too many nucleations around the
edges. IIRC, they only occurred rarely and when you did something wrong.
In fear that the copper might be contaminating the Bi, I’ve been playing with glass lately. I kept getting nucleations because the oxide made the
metal stick to the glass. Basically, small vibrations made the melt creep a bit above the meniscus, stick there, and cool disproportionately quickly,
nucleating the melt when it wasn’t supposed to.
I then started coating the inside of the beaker with a thin layer of plaster, but this made things worse. The melt doesn’t stick to the plaster, but
the <b>entire</b> plaster-bismuth boundary nucleates at once. The only major difference between the plaster and glass/copper is that the
plaster is somewhat porous due to loosing its water of hydration at these temperatures. Maybe someone here with more crystallographic knowledge than
me can explain this? I’m stuck up a wall on this one.
|
|
|
| Pages:
1
2
3 |