| Pages:
1
2 |
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Disposable Argon gas cylinders
Yeah,.....you are right about the streets here. There is so much dogs shit laying around that you can actually slide through Amsterdam. And about the
ripoff of foreign visistors, well,..... actually we like to rip each other off down here. And the women....., now back to Argon.
The dispossabel cylinder I linked to, costs 11.5 pounds and contains 300 grams of Argon gas. The price is rather high, so you will be able to find it
a lot cheaper ellswere.
[Edited on 28-6-2005 by Lambda]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I also hear that area (can't remember if it's Denmark, Sweeden or what) has massive unenployment, suicide rates and heavy taxation... strike
one up for socialism eh.
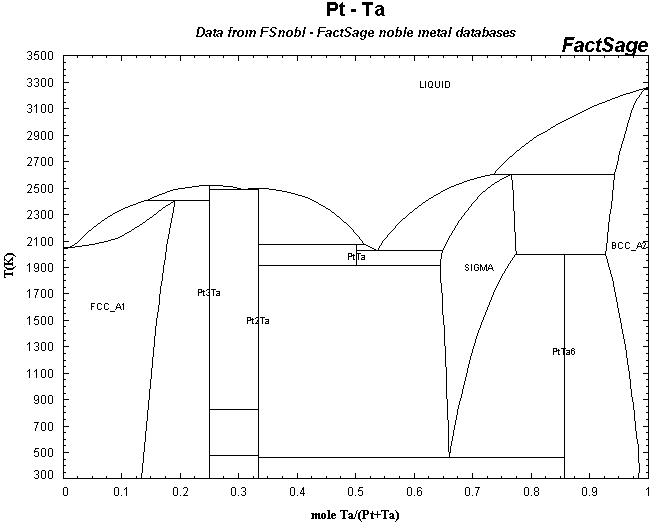
Anyways, here's the phase diagram. I don't have Pt-W in FactSage but it's on the site linked above.
Tim
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Well, I fail to grasp any information from these. Where does the 1100*C number in the patent figure in these graphs? What's SIGMA in the Ta
graph, and what about the unmarked regions at the bottom? The W graph seems incomplete, in that some regions are not fully delimited (2, 3, 6).
And what about that finishing.com comment I referenced? Overall, I don't know anything more about modifying the procedure from the patent for W
than I did before starting the thread...
And regarding socialism, let's look at the CIA's World Factbook. Annual GDP growth rates for 2004 of stable economies (that is, developed countries, otherwise the comparisons are meaningless):
Capitalist USA -- 4.4%
Socialist Germany -- 1.7%
Socialist Italy -- 1.3%
Socialist Netherlands -- 1.2%
Half-and-half Canada -- 2.4%
See a pattern foks? Kill pinko bastards!
Luckily for the EU, things are starting to change. Look at Germany, for example, with the oncoming shift to the right. But I'm worried about my
dear Canada, what with the current rise of commie bastard Layton and his cronies.
[Edited on 28-6-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Diffusion is a different thing... AFAIK, you heat to increase solid-state mobility and solubility. You can take aluminum butted against magnesium, at
room temperature it won't really do anything (I heard there's a "2000 year sculpture" somewhere that, in 2k y, will diffuse at
room temperature into MgAl intermetallic - if it doesn't break before then!), at elevated temperature however it'll diffuse together and
whatnot in a reasonable time span (hours).
Ceramics take advantage of this. You can fire just about anything quite nicely without a liquid phase.
About the Pt-W diagram: it might continue towards room temperature with typical decreasing solubility; it may also instead transform into
intermetallic phases (gamma and epsilon are mentioned but not indicated; the scale also stops at 1000C). This could be like Cr-Fe:
http://web.met.kth.se/dct/pd/element/Cr-Fe.html
Which as you can see, forms a sigma phase momentarily before excluding solid solubility. Fe-Ni and many other transition metal diagrams have similar
discontinuities. It is probably inconsequential, certainly if you are going to diffuse above that temperature. Have fun at the periodic table on
that phase diagram site too, BTW.
As for Pt-Ta, it looks like sigma is PtTa2, since it ends at 66.6%at Ta. Diffusion between solid blocks would form many, probably brittle,
intermetallics in the joint.
If Pt is a thin layer and diffusion is conducted for a long period, there won't be any pure Pt remaining. That's probably (outside) your
upper time/temp range. Too little "heat work" and it doesn't diffuse enough. There's also the issue of the Ta oxide layer, which
I'm guessing must be overcome by thermal energy. W has an oxide layer as well, but being less reactive, it's much thinner.
Tim
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
So should I initially try the same temperature/baking time for Pt on W as was used for Pt on Ta in the patent? I can always plate a second layer if
too much diffusion occurs, but the problem is that the only way I'll know it it's too little is if the Pt doesn't adhere, which means
testing by trying to scrape off the plating -- which sucks as it wastes epxensive Pt.
In that finishing.com comment, it says to dissolve the W oxide layer in Na2S2O8/NaOH solution. Can I make Na2S2O8? It's not locally available,
as the PCB etchants here are either FeCl or a mix that contains Na2S2O8 but mixed with other shite I wouldn't know how to remove.
[Edited on 28-6-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Electrosynthesis of persulfate
Electrosynthesis of persulfate:
If you are interested in making it by means of electrosynthesis, then much more info can be found, including purifying what you allready have into
pure Sodium persulfate.
US Patent 4802959 Electrosynthesis of persulfate
http://www.freepatentsonline.com/image-4802959-1.html
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
So electrolysis of H2SO4, then addition of NaOH should do it. How do I split the cell, if I don't have an appropriate membrane?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Texium
|
Thread Moved
19-11-2023 at 10:39 |
| Pages:
1
2 |