| Pages:
1
2
3
4 |
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Your nose can be used for you, or against you
| Quote: | Originally posted by neutrino
Actually, mine has been improving since I stopped playing with chlorine. 
|
You should try a cup of coffee after smelling Chlorine gas, it tasts like sh**. Have you ever smoked a cigaret after a sniff of Phosgene, it tasts
like Metall.
If you want to smell what a bottle contains, then the easiest and safest way to do this is: Unscrew the bottle or container, hold the cap or lid about
50 cm from your face at chest hight, and with a slow motion, wave air it towards your nose. If the smell is to faint, then gradually bring the cap
or lid towards your chest, and repeat the same movement.
Never smell a container or bottle directly through the nek, people have severely damaged there sineses by doing this, or even worse. Many of you have
most probably had bad experiences with concentrated Ammonia.
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
heck ammonia is too much!
Yeh 28% ammonia can throw you down on the floor! One wiff sent me into a lacrimation fits. The deal was I was reacting the ammonia with a bromine
solution in HBr and I added exess ammonia to discharge ALL the deep red color since ammonia reduces bromine to ammonium bromide and nitrogen gas in
aqueous solution. Thinking the ammonia was oxidized I leaned closely over the bottle. Luckily I did not drop the flask on my feet! After everything
I did get about 80 or 90 grams of pure white ammonium bromide though! 
Lamba, if you don't mind yellow skin, 70% nitric acid works with one application! Stings a little though.
Oh about 10 years ago i worked in a metal shop and there was a white brazing paste that contained potassium biflouride. I accidently got about 2 or 3
grams on my bare hand while cleaning some tubing. YEOW! This hurt much worse that nitric acid. Think 1 million microscopic razor blades! That how it
feels! I know I want to use bifluoride salts to do titanium and niobium work but I really have to watch these caustic salts!
[Edited on 8/21/2005 by chloric1]
[Edited on 8/21/2005 by chloric1]
[Edited on 8/21/2005 by chloric1]
Fellow molecular manipulator
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Chloric1, indeed, Hydrogen flouride and Bifluorides are often very much underestimated in there effect on the skin. I had mentioned Hydrogen flouride
a few posts back in: Looking for pain and scares ?. Intoxication by flourides, is about as painfull as it can get, you would rather rip your stomach
out, than bare anymore pain.
Be greatful, for being hit by Ammonia, and not by Hydrogen azide. This one sends you down so fast, that you literaly crack open your scull when
hitting the floor, only after you had first slamed it against the lab table. It dose the same as you would expect from Hydrogen cyanide, only Hydrogen
azide first slaps you real hard in the nose before you come crashing down. The smell is intollerable and evil.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Chlorine is my worst enemy, and the reason I'm breaking down and getting a gas mask after one to many leaking chlorine generaters and the pain my
sinuses endure every time I use it. I have noticed my sense of smell is deadened after using chlorine gas for some time, but since I can't
really compare it easily I'm not sure how much it has actually deteriorated.
At least it's nice to know that it will begin to heal over time....
| Quote: | Originally posted by Lambda
Have you ever smoked a cigaret after a sniff of Phosgene, it tasts like Metall. |
It seems that if you could market this as a 'quit smoking now' type program, you would make millions before the lawsuits arrive. By then
you would be in Mexico 
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I agree with Lambda, HF would definitely be a bad bad experience. Not only would it hurt, I'm nearly positive that it is a potent poison that
really messes with the skeletal system. I know for a fact that many colleges have gradually removed HF from their stockrooms because of the hazards
involved in its use.
I've never experienced hydrogen azide (would be an interesting synth I'm sure) but I can tell you that hydrogen selenide is horrible! The
stink is terrible and if you get over exposed, you're sick for months.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
| Quote: | Originally posted by Fleaker
...I'd rather have sulfuric acid spilled on me than hot elemental bromine. That takes months to heal... |
My thoughts on this:
elemental bromine + skin: bad
hot elemental bromine + skin: YEEEEOOOOOWW!!!
| Quote: | Originally posted by Fleaker
...Fuming nitric is pretty bad too, stains your skin mustard yellow... |
vulture gave a nice explanation on the wonders of "nitroskin" in a Whimsy thread
| Quote: | Originally posted by Fleaker
Just goes to show that being a chemist has its dangers :p! |
Samosa says:
| Quote: | Originally posted by Samosa
Indeed, many of my pets are dangerous; but as is always the case, "the hydrogen fluoride is just as afraid of you as you are of it." This
can be said about any chemical, I'm sure. Perhaps they just seek to protect themselves from ones who cannot manipulate them properly?
|
| Quote: | Originally posted by Fleaker
anyone else here notice that their sense of smell is deteriorating? 
|
Funnily enough, after all the years I've spent in the lab with nasties, my labmates still use my nose as a makeshift chemical analysis device.
 
| Quote: | Originally posted by Lambda
Never smell a container or bottle directly through the neck |
Hell no, that's suicidal!
| Quote: | Originally posted by Lambda
Many of you have most probably had bad experiences with concentrated Ammonia. |
That's why you're supposed to use moist red litmus paper instead of your nose when detecting ammonia. 
| Quote: | Originally posted by Lambda
Be greatful, for being hit by Ammonia, and not by Hydrogen azide. This one sends you down so fast, that you literaly crack open your scull when
hitting the floor, only after you had first slamed it against the lab table. It dose the same as you would expect from Hydrogen cyanide, only Hydrogen
azide first slaps you real hard in the nose before you come crashing down. The smell is intollerable and evil. |
Rosco Bodine had this to say about hydrazoic acid:
| Quote: | Originally posted by Rosco Bodine
...such solutions are an extreme danger due to the toxicity and volatility .
The material is so toxic that it has been said if you smell it , then you have
already been poisoned . And that is not very much if any exaggeration .
Extreme Caution is the imperative rule for hydrazoic acid . It simply is not
the sort of material inclined to suffer fools or carelessness without exacting
a dear price for the error . It is among the deadliest toxins known to science. |
| Quote: | Originally posted by Chris The Great
It seems that if you could market this (phosgene) as a 'quit smoking now' type program, you would make millions before the lawsuits arrive.
By then you would be in Mexico |
Their lungs are on the road to total deterioration already, so why should they complain? 
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
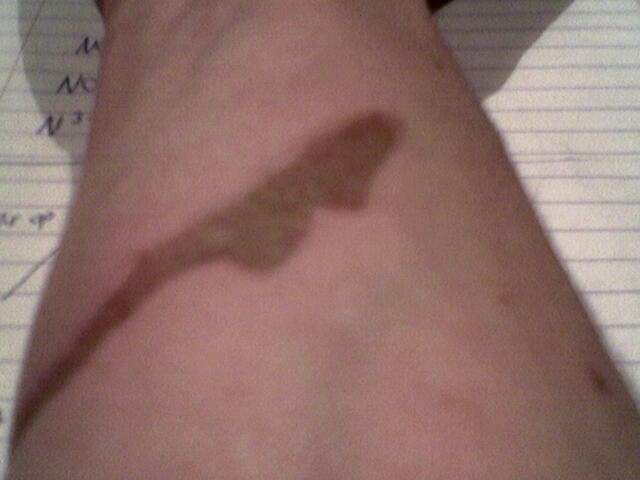
Another acidburn
Again with conc. sulfuric acid. I let it sit for 2-3 min. until I washed it off.
After having my arm under the cold tap for servel min. I could peel the skin right of and underneath the skin is kinda seethough for 1mm or so.
I Know this has nothing to do with chemistry and I should be in therapy... and I am.
|
|
|
denatured
Hazard to Others
  
Posts: 151
Registered: 7-8-2004
Location: -
Member Is Offline
Mood: HCl 50%?
|
|
LOL, i think this thread fits best in whimsy forum... i didn't know that there are a lot of mad scientists out there ... 
This is an entertainment thread although that horrible accidents you encounter ...
I always keep water and milk handy just in case.
i used to inhale HCl from h2so4 and salt and it is quite refreshing
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
If you go swimming in an over chlorinated swimming pool and have a cigarette afterwards you can taste phosgene.
mick
|
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
| Quote: | Originally posted by Lambda
I also once had a wart, and just like you, I got fed up with the normal treatment. When I applied Silver nitrate (hells stone, which decomposes under
the influence of light, and produces Nitric acid in situ) or formula W (I think Salicylic acid) the wart just became bigger or nothing happened. So in
desperation I went into the lab, and got hold of liquid Nitrogen. I poured the liquid Nitrogen out of the main tank into a Dewar vessel, but because
of all the white clouds, it is hard to tell when this vessel is full. A lot of cold gas moved down onto the floor of the 5 * 5 meters filling room,
and it was covered by a 20 - 40 centimeter thick cloud blanket. It's quite scary, for you canot see the floor anymore. When I walked away, I heard a
loud cracking sound. You have quest it allready, I split my rubber shoe sals in half. Both my new shoes where f****d up. So, having been hit by this
rather unpleasant experience, I became cautiouse. I only applied a little Liquide Nitrogen by means of a cotton wool stick onto this wart. And you
know what happend, it didn't go away and instead of being 0.5 cm, it became nearly 2 cm. It somhow had spread out underneath the blister. This took a
wile though to develope. I got so pissed off, that I again applied liquid Nitrogen, only not in a cautiouse manner. I had a hole on the palm of my
hand about 8 mm deap, and 3 cm wide after the blister had been cut open. After having applied a strong antibacterial ointment, the wound was closed
with cloth soaked in the same, with a plaster to hold it in place and keep out dirt. I am now looking at my palm, seeing no scares and not even the
slightest trace of this gaping wound. I realy thought my hand was going to rot off, it looked that bad. [/quote
I can remove warts in minuts! After I got result,I was left wonderin' Why surgical excision Is still the tecnique in vogue when immediate result is
required. Which is done by nurse,not surgeon! It does not work most of the time the wart regrow.(to disappear after 2 years max most of the time
I use a combinarion of acids and base,follow 2 oxidizer,follow meccanical extraction of the bits/roots (forgot proper name now) I pull the lot out.No
roots left!
Also there are good result using an acid present in woman
breastmilk. I will post ref. if required.
And ammonia or bases will defatten the derma layer. Did not see it in this tread
[Edited on 16-1-2007 by gil] |
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I guess I am fortunate. In 40+ years of experimental chemistry I have never had a serious acid burn. Worst have been a few ruined clothing articles, a
few brown nitric acid stains that peeled off.
I was the designated disposer, down the drain in a hood, of excess inventory of chlorosulfonic acid, fuming sulfuric acid, fuming nitric acid,
sulfuryl chloride etc. for the FDA lab in New Orleans, my boss from the University consulted for them and when they had a dirty job to do like this, I
got to do it. But I gloved up and goggled up and lowered the sash. No worries.
At present have 4 liters unopened 65% Merck fuming sulfuric on hand. Some chloroacetic, to make into chloroacetyl chloride. Some oxalyl chloride. Some
chlorosulfonyl isocyanate. About a Kg bromine. These need to be respected or else.
|
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
65% Fuming sulfuric acid?I tought Fuming is 100% + oleum added into it And 65% is diluited. I have
2.5 L. 99% sulfuric acid and would not call it fuming sulfuric acid.
|
|
|
unionised
International Hazard
    
Posts: 5103
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
H2SO4 + 65% of its weight of SO3 is very clearly fuming sulphuric acid.
|
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Got it now.It wasn't clear to me(65% fuming H2SO4) I tought was 65% : 2/3 total ratio.Thanks.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@unionised is quite correct.
It is 100% H2SO4 +65% by wt SO3
Primarily used in microchip production.
Most lab fuminf sulfuric is 20-30% SO3. (One of the other, not so vague a range.)
I paid a pretty penny for this Merck product, given that the importer and reseller are greedy, about 2X the Merck ex works price.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I should mention that where I live, the govt restricts import of SO3 as such, chlorosulfonic acid, fluorosulfonic acid, sulfuryl chloride, sulfur
dioxide, etc. one must obtain a permit from the Ministry of Defense to import, manufacture etc.
But fuming sulfuric acid is under no such restriction.
Furthermore chemical importers need to have import licenses on a compound by compound basis and not all of them have licenses for all of the products
their parent companies sell. Merck Thailand has been around longer than most and has apparently done a more thorough job of obtaining and maintaining
the validity of such licenses, as compared with Carlo Erba, Panreac, Acros etc. Local stocks are not very comprehensive, and most things have to be
ordered in, meaning delays and snowballing shipping fees.
Recently I wanted some chloroacetyl chloride. Only one company had import permit for this, and upon ordering I was informed that shipping regulations
forbid air transport, and that surface transport regs now require a refrigerated container. The sum of all that was NO SHIPMENT.
Well now I have 2 Kg chloroacetic acid at far lower cost and the means to make the acid chloride from it. Unpleasant stuff.
Life's a bitch.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I would really like to have some Oleum as well (the 65% kind, not the lame 20% kind). Oleum is one of the few chemicals that I simply refuse to buy
because of its horrendous price. I will not pay well over 100€ for a liter.
How much did you pay for it, Sauron?
Chloroacetic acid is also extremely hard to get here, because it is "toxic". I am however planning to make some myself, by chlorination of GAA in
presence of PCl5.
|
|
|
Misanthropy
Hazard to Self
 
Posts: 69
Registered: 24-3-2005
Member Is Offline
Mood: Variable
|
|
I get a vibrant, rainbow colored, kaleidescopic dermal psyche warp.... not to mention the hair!!!! oh..
|
|
|
unionised
International Hazard
    
Posts: 5103
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I think that may be the effect of "acid" on some other part of the body.
|
|
|
ac-
Harmless

Posts: 6
Registered: 21-3-2007
Member Is Offline
Mood: No Mood
|
|
Hydrofluoric acid on skin!
http://content.nejm.org/cgi/content/full/356/6/e5/F1
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I must be either Fortunate or carefull, I have many of the acids mentioned and at the same concentrations, as well as the Bases and the Br2 liquid.
I`ve never been burned yet happily, and hope to stay that way.
although I did have a hole in some rubber kitchen gloves whilst cleaning and oven and parts out with NaOH soln, never felt any pain at all, but my
finger nail turned into a gel that could be scrapped off, and when dried it went yellow and cracked 
I should have used my Lab gloves and not trusted domestic kitchen ones (totally my own fault).
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
WOW! He survived!
Tim
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Reading stuff like this really affirms my being a bit of a pussy when handeling nasty shit like F3CCOOH, (conc.) H2SO4, conc. HCl, Br2 and HNO3. The
only thing I might need to be more careful with is LN2 but that's just too much fun. (not to mention being a complete pussy around strong solutions of
NOCl and (Na,K,Li)OH)
How do you guys normally approach such materials? gloves?
-Edit- JESUS FKN CHRIST! He had the calciumcrap coming out of his skin...
[Edited on 30-7-2007 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Nerro
How do you guys normally approach such materials? gloves?
|
Latex gloves, usually. I used to not bother, but a well nitrated index finger a couple years(2?...3?...) convinced me to start. This summer the skin
on that finger seems drier(and kinda cracked in places) than before...hmm.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I get boxes full of latex free Nitrile disposable gloves, and even then I handle the material as if I weren`t wearing gloves just to be on the safe
side.
also, most of the time the acids I use are dilute also, there would be no advantage in using super conc acids, in fact I`ve found that for a few cases
only Dilute will actually work 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
| Pages:
1
2
3
4 |