| Pages:
1
2
3
4 |
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I usually prefer to use nitrile (latex causes redness and little red-spots that feel like a pin is sticking in them--allergy), but...I have noticed
that nitrile nitrates very nicely, turning a bright yellow before it fails (so, at least there is some warning). I have noticed that conc. H2SO4 gets
hot when in it gets onto latex gloves, before they fail; again a warning.
What's bad are the things that penetrate without warning. Cinnamoyl chloride (and likely otherr aromatic acyl chlorides) will go through nitrile where
they proceed to cinnamoylate and dissolve your skin (HCl made with the water in your skin). Nice.
Acetic anhydride inside of you glove (unnoticed pinhole) is also interesting. No pain, but when I took the glove off, my left index finger was "corpse
grey" and wrinkled. I stuck it in the base bath and rinsed thoroughly with water (until my skin tested neutral to litmus). Still, no pain, but a day
or two after, all of the skin on the hand dried out and peeled off (like sunburn).
TFA is nasty (but the "smoke" is quite cool, you feel like a mad scientist just opening it). I handle it very carefully, and have had no permeability
issues or burns.
Pyridine and the like also penetrate nitrile (for reference).
When I was a metals analysis tech (nitric, HCl everywhere), I did not have a piece of clothing without a hole in it, and my fingers were always yellow
(particularly the index and bird finger where I would hold the stoppers whilst sampling from volumetric flasks). An errant drop of HNO3 (70%) on the
forearm*, unoticed produced a deep orange pit with a yellow ring, and felt like being jabbed with an icepick.
The fluoride method in boiling conc H2SO4 is bad too. No matter how hard you try, a drop always gets on you somewhere. Move calmly (but quickly) to
the sink! Small burns (1-5mm, maybe, from the droplets) resembled broken gears's.
The worst, though, I think, is hot 50% NaOH. It burns like hell, immediately, and is difficult to wash off. The grease on my face actually protected
me wuite a bit though--There were no markings there, but on my neck and forearms*, wheals. Yowch.
* I wear a labcoat, but I roll the sleeves up to my elbows. I found out the hard way that a drop of something on your forearm sucks, BUT, a cuff
soaked in something and then unwittingly left in contact with your wrist is much, much worse.
Be careful,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
feacetech
Hazard to Others
  
Posts: 163
Registered: 12-2-2007
Member Is Offline
Mood: No Mood
|
|
glacial acetic acid gives the oddist of burns
I spilt some on my finger tips, didnt notice much and washed it off.
After a few days it looked as though there was black brusing under my skin my fingers felt very tight like they were going to burst out of the skin
and were very dry feeling I had to constantly lick them to relive it. It slowly got better but lasted about two weeks
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
I prefer reusable, thick, glove that go up to your forearms. I try to be environmentally conscious. (considering how un environmental the TDS (total
dissolved solid) levels from my household water must be)
Chemkid
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
A long time ago I was boiling concd. H2SO4 and shook the liquid on accident, a small amount of the acid overflowed. I didn't notice this until about a
drop of it hit my skin and was extraordinarily painful I looked at my hand where the pain was and the skin was dissolved. Although it was most
intense, strangely (thankfully) though the pain didn't last long at all.
If you want to test the corrosiveness of a substance on something closest to skin, then meat should work.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by feacetech
glacial acetic acid gives the oddist of burns
I spilt some on my finger tips, didnt notice much and washed it off.
After a few days it looked as though there was black brusing under my skin my fingers felt very tight like they were going to burst out of the skin
and were very dry feeling I had to constantly lick them to relive it. It slowly got better but lasted about two weeks |
I've spilled glacial acetic acid on me on accident and it stung pretty bad.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I spilled an entire quart of nearly boiling hot, concentrated H2SO4 on the floor where it splashed on to everything (including my jeans).
Surprisingly, none of it got through to my skin (my pants were kind of baggy). It just made my pants look like someone threw hot coals all over them.
One of the sensations I hate the most is a 35% H2O2 "burn". It turns your skin completely white and you get this "tingling/burning" sensation at
random times until it heals. I hated that sensation!
Other than the H2O2 I have been fortunate enough not to have any sorts of burns (except a little NaOH). I guess I'm just very lucky or very careful.
Well, I've probably had some other kind of "burn" that I just can't recall at this moment.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
The H2O2 burn is annoying.
The worst I have ever had is molten KClO3 spilling on my skin. It was an interesting feeling, your skin being used as a fuel to burn itself even more.
When it was over, and after I picked the nice carbon chunk out of my skin, it left an odd looking and painful scar with lots of little carbon
fragments stuck in it.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Slightly offtopic, I once got a bromine burn. Took 2 months to *kind of* fully heal; the pain was excruciating for days. The burned-up-by-bromine
tissue stinks in a horrible way; something between rotten flesh and strong chemical smell (close to bromine obviously).
Once on the skin, it took like 2-3 seconds before the bromine literally got SOAKED into the skin like it was on paper. Then I felt the burn; nothing
helped (oil, water, you name it).
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
The most amusing had to be a litre of 20% HCl pouring from a catastrophically smashed container into my LAP. I was sitting at the lab bench in school
holding the big glass beaker, waiting for a friend to get the container for me to decant it into. Some idiot carrying a retort stand accidentally hit
the stands 'pole' off the beaker, which suffered instantaneously effective catastrophic structural failure (i.e. it shattered), depositing the entire
contents onto my lap - and my bollock region! Fearing that the meat and two veg would be dissolved into so much chemical soup I grabbed someones
carefully prepared 500ml beaker of Na2CO3 solution and dumped it on myself, prior to pouring liberal amounts of water on the affected area of myself.
Thankfully, only my thigh region got a minor rash and my pants began to degrade every time they went into the washing machine as their structural
integrity was compromised. ALl in all a VERY amusing accident.
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | I was sitting at the lab bench in school |
So make up your mind, are you a rather well known
chemist, or a high school student? Most chemists wouldn't hold a beaker of acid over their lap, would they?
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
*grin* Well... Lets not discuss the personal matters but I am currently trying to file several patents on my blasting caps (caseless) and blasting
compositions for oil wells.
I am young, but then again I started very young. I am proud to say that in this day and age it is my generation that is innovating - one man I respect
is not much older than I am, and he is VERY well known in the online community - I see him as a drug chemistry/ botanical genius.
By well known it depends on what circles you frequent. Try... Roguesci. I was lurking since 2006, yet only joined in 2007 and begun in earnest in '08.
A steep learning curve for a young man with upcoming state exams but by mid '09 I was trying to sell my ideas. By the end of 2010 the projected pplan
is to get the things patented at last, and start business as by then I will be done with my second level education and well into third level (Uni).
Youth is NEVER an indication of ignorance, nor is age an indication of wisdom. When, aged 16, you find yourself permanently scarred and damaged, in an
operating theater, due to inferior energetic materials, you grow up fast. You rapidly become more obsessed than Davis ever could be, and find your
innovative side - that is, if you are not scared off the scientific pursuits for life.
While not yet published in the literature, rest assured that I am (allegedly) known in the community of AMATEUR researchers as somewhat of a mixed
persona - willingness to put self in harms way suggests idiocy, yet the samples of innovation and invention suggest otherwise. If only I could put an
end to the fucking idiots emailing me stupid questions...
I have great respect for the wise and knowledgeable people here - they are the type of person I aspire to become.
But you sir, you appear to have some vindictive side in you - protected by the anonymity of the Internet - you embark on what you see as 'idiot
bashing' or 'flaming'. Rather immature - the type of thing I expect from First Years in schools (13/14 y/o people). Please, let us put this pathetic
quarrel behind us and behave like proper gentleman/ scientists on the pursuit of knowledge.
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
More on the original topic: I once spilled a flask of aqua regia on my hand - luckily it was somewhat depleted since it was being used to dissolve
gold-bearing metal powders. It felt hot (it was at least warm from the reaction) in the 5 seconds or so it took to get it under the cold water faucet.
For the next couple of months, my hand was purple (purple of Cassius, prepared in situ  ). It slowly flaked off.... ). It slowly flaked off....
I must have pretty tough hands - the standard acids (hydrochloric, nitric, sulfuric) either concentrated or dilute don't do much if I wash very
quickly. Alkali, though, I'm -really- careful with. Picking up glowing pyrex with bare hands must do something to toughen the skin. Occasionally it
causes the brain to go into gear, but not often enough.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Picking up glowing pyrex with bare hands must do something to toughen the skin. |
You're in good company.
Stories about Bunsen say he could take the top of a red hot crucible without using tongs. One of his students said he'd often smelled "burning Bunsen". One of his students said he'd often smelled "burning Bunsen".
|
|
|
Popi1955
Harmless

Posts: 2
Registered: 9-8-2009
Member Is Offline
Mood: need to blow something
|
|
i havent got the chance to try strong acid since all i got is hydrochloric 32% and hydrogen peroxide 35%, the hydrochloric is lame, if i havent cuted
my skin it would not affect undameged tissu, the hydrogen peroxide is much much more powerfull then the acid, well, it still dosent burn u with
contact with your skin, but it can make burns on undameged tissu after few mins, the burns were not so deep, so i decided to try mixture of hydrogen
peroxide the hydrochloric acid, the hydrogen peroxide makes the hydrochloric acid to decompose into hydrogen and chlorice, witch need to be more
corassive, it still dident had much affect, so i found out that if it used on dameged tissu the affects and faster and much stronger, no need for the
tissu to be open, like a deep cut or something, a scratch is enough, i stiil have on my hand unheald wounds, i did it about 3 weeks ago, one of the
wounds looked like a hole in my hand, i still havent got to try stronger acids, or sodium hydroxide that i would lovely try, ill upload photos
someother time, sorry for my english
|
|
|
MattVon
Harmless

Posts: 5
Registered: 25-7-2012
Member Is Offline
Mood: No Mood
|
|
I believe that the free H+ ions in an acid solution hydrolyzes (break down)
the proteins and cells on your skin. Some acids like sulfuric and phosphoric acid also have dehydrating properties, meaning they suck the water right
out from your cells, and they can result in thermal burns.
My first burn was from sulfuric acid about half a year ago. Ate straight from my right index finger to nearly the muscles, and left a scar. And since
then I was a little bit paranoid of acids. I wore ridiculously heavy-duty gloves and safety gear.
I also had worked with hydrochloric, acetic, formic, and a bunch of other acids. I've been also sometimes been working with hydroflouric acid in the
lab, but I'm not coming close to that terribly dangerous stuff after my first acid burn..
[Edited on 26-7-2012 by MattVon]
|
|
|
mineralman
Hazard to Self
 
Posts: 64
Registered: 15-6-2012
Location: WALES UK
Member Is Offline
Mood: complicated
|
|
When I was but a mere teen, I decided to boil down a 3ltr pan full of old filtered battery acid on my oil fired aga top. I was unaware that the
casserole pan I used wasn't to be used str8 on to such a high heat.
YEP you know what comes next, I watched as it shattered/exploded into a huge cloud of white bellowing acid vapore. I swear it shattered in slow
motion, I can still picture the fragments of the pan flying in all directions and being replaced with this never ending cloud of acid fumes.
I only got a few splatter burns fortunately, the house however, was far less fortunate then I.
My initial reaction was to exit the house into the courtyard/beer garden, ( the house was an old 14 room pub/grub & rooms to rent ), What I
learned that day about acid fumes, the never ending white smoke that came off of that contsantly hot stove went on for the best part of two hours.
I was outside for 1/4hr before I realised this was not going to be over soon, and that I would have to return to open all windows, doors &
skylights. 4 in/open everything in the room/out stealth operations later, id opened everything in 8 rooms on 3 floors, chokeing, tears stinging my
eyes and almost passing out twice, all I could do was watch is horror as half the house was bellowing out white smoke, crowds of neighbours started
collecting and laughing out " what did ya do this time?" man oh man what a mess 3ltrs of dilute sulphuric acid can do to a houses decore.
I hate to think of the mess I could/would have been in if it had splattered just that little bit further.
I now have a healthy fear of acids and put 5 layers of clothing, full acid gauntlets, face shield & buckets of neutralising solution on hand, just
in case. MM
|
|
|
JeanHarris
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
That's good precaution !
I just want to say that when I was swimming in my pool with swim fins, the chlorinated water had gone reacted with my fins and I got weird type of
allergy. Why this happened so? Any ideas?
|
|
|
Antimatter
Harmless

Posts: 4
Registered: 26-7-2012
Member Is Offline
Mood: No Mood
|
|
Acid simply burns your skin by breaking down the proteins, lipids, and fats that constitutes your skin. This is often called hydrolysis. The exact
concert of how this works is a little complicated, but in laymen's terms, a cell-seeking agent like the H+ ions in an acid solution attacks the
carbons that make up skin cells, therefore damaging them. Bases work the same way, however they tend to be more insidious than acids. Bases slowly eat
away at your skin, while acids attack so fast you can feel it.
One of my most interesting acid accident is about two months ago when I was working with some highly concentrated (about 36%) hydrochloric acid. I was
carrying it in an Florence flask, but I was a little too hasty in setting it down. I placed the flask quickly on my worktable so I can reach for my
other reagents, but I placed the flat bottom towards a too sharp an angle and it tipped over and spilled the acid all over my pants and part of my lab
coat. Instinctively, I grabbed a bottle labeled sodium bicarbonate and dumped it onto the splash area.
Dumb mistake, because I was a rather sloppy chemist. I had accidently labeled a bottle of sodium HYDROXIDE as sodium bicarbonate. The exothermic
neutrlization nearly burned me, and now I have two caustics on my pants. So I ended up with a bad rash on my legs and a wasted pair of pants, but I
counted myself pretty lucky for that one.
|
|
|
triplepoint
Hazard to Others
  
Posts: 127
Registered: 11-4-2012
Location: U.S.
Member Is Offline
Mood: in equilibrium
|
|
Many years ago (in school lab), I spattered some HCL on my leg. I didn't notice it until I saw the holes in my pants. Being a stupid kid, I didn't
realize what happens next. When I later took my pants off, I observed several pits burned into my leg. Luckily, it was not more serious than that.
This is slightly off topic, but it was not my first mishap with chemistry while in school. When I was about 10 years old, I picked up a lit alcohol
burner. It tipped and set my arm alight. Lucky again, the alcohol burned off without even making my arm hot.
Even farther back, I managed to cause a classroom to be evacuated when I demonstrated a volcano project. The volcano was clay with a pan set in the
top for ammonium dichromate. When I lit the dichromate, it ignited the newspaper I had used to stuff the clay volcano. The flames then caused the
clay to explode. Now that was a volcano!
This was all 30-40 years ago. I am considerable more circumspect and careful these days (otherwise I would probably not be able to write this).
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Formic acid did a nice little job on my hand. The tip of a pipette with a residue of 88% formic acid touched me, it started to burn pretty badly after
about 5 seconds, it was washed off after 10 seconds. Minutes later the skin was peeling, the next day is this- ouch.
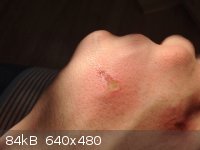
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
I've run into an interesting effect. After repeated exposure to various weaker solutions of acids and alkalis, the skin on my fingertips becomes
thicker, horn-like and somewhat microcracked. In this state, my fingertips appear to be immune to everything less bad than 95% sulfuric. Steaming and
rubbing appears to remove the horn layer from the skin, and the immunity goes with it.
Smells like ammonia....
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The same happens with physical abrasion/pressure on a daily basis.
The dermis builds up into a callous, which is basically loads of dead tissue between the outside world and your more important tissues.
Expendable skin effectively.
If you remove the repeated need for it, then it goes away.
If you're carful to avoid the underlying tissues, you can cut chunks away with a scalpel (wear safety goggles in case you go crazy and hit an artery).
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
The other day we were calculating the dissolution heat for benzoic acid in an experimental physico-chemistry class and my partner managed to shatter
the titration apparatus (containing NaOH) while I was agitating the benzoic acid. The basic spray went all over my hands and on my coat on the chest
area. I washed the base off my hands and finished the experiment, after all this thing took long enough and I was hungry as heck. When I got home I
noticed a slight irritation in my skin but nothing major. And that was the closest I got from a chemical burn.... See a chem. E. Undergrad life is
boring even when lab accidents happen... 
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
I don't know if this link will work for everybody, but the story is relevant as well because it is leading to the restriction in sales of chemicals.
I can't organize well what I think about the story so I'll just post the link.
http://www.nytimes.com/2015/09/10/world/asia/with-red-lipsti...
Any other SF Bay chemists?
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
In the university a friend of mine studies, a freshman had retinal detachment because another student assaulted him with acid. Obviously it wasn't
concentrated H2SO4, otherwise he'd have lost his eye... and the assaulter was someone with access to analytic reagents in the university labs.
And I had no point in sharing this other than saying that it also happens outside India.
IMO, crazy people will manage to hurt others no matter how much we limit access to "dangerous stuff". As someone once said: "A lock only serves to
keep a honest person out". Even with the restrictive weapon laws where I live, punks still get guns and shot each other in night clubs. Even if you
need to be 18 to drive people in their late 40s still run over and kill bikers. Restricting chemicals because they can be used to harm people is more
or less the same as wanting to restrict the selling of wood saws and kitchen knifes.
Sure this is the opinion of a home chemist who doesn't want the government to take his toys away... soooo yeah, not exactly unbiased.
|
|
|
| Pages:
1
2
3
4 |