cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
modified Wohl-Aue reaction?
The wohl-aue reaction is where nitrobenzene reacts with aniline to form a substance depicted in the following link:
Wohl-Aue reaction
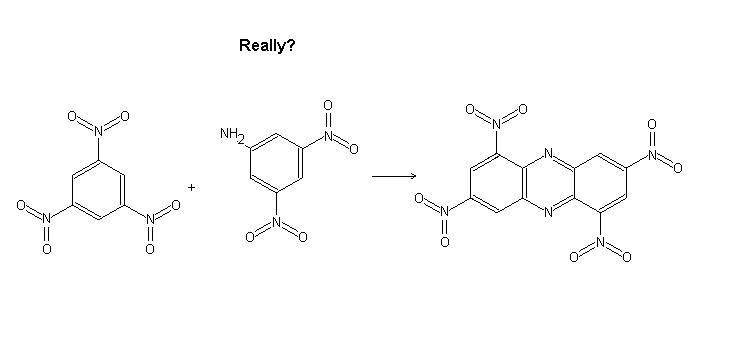
I was wondering if it were possible to substitute nitrobenzene with 2,4,6 trinitrobenzene and aniline with 2,4 dinitro 6 aminobenzene. Would this
work? The product would be interesting.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Likely impossible with 2,4,6-trinitrobenzene and 2,4,6-trinitroaniline, but with 2,4-dinitrobenzene and 2,4-dinitroaniline it could potentially work.
With the 2,4,6 trinitro compounds there is no empty sites for bonding to occur. Nitration of the end product could be interesting as well.
|
|
|
cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
Whats the name for the original (unnitrated) substance? I couldn't figure it out.
|
|
|
cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
Really- no room to bond? I thought there was...
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
| Quote: | Originally posted by cumbustion
Whats the name for the original (unnitrated) substance? I couldn't figure it out. |
That would be Phenazine. 
Edit: Haha Axt, I was first 
[Edited on 28-8-2005 by Joeychemist]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by cumbustion
Whats the name for the original (unnitrated) substance? I couldn't figure it out. |
<a
href="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13537156&dopt=Abstract">Phenazine</a&
gt;
EDIT: Damn you joeychemist  I forgot to type my password in the first time too. I forgot to type my password in the first time too.
| Quote: | | Really- no room to bond? I thought there was... |
3,5-dinitroaniline has a of lot better uses then that!
[Edited on 28-8-2005 by Axt]
|
|
|
cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
Would it than be a waste to start a thread on tetranitrophenazine or similar substances?
What are the exact conditions needed to bring about a wohl aue reaction? Or for that matter my modified one.
The two nitrogens in the middle of phenazine -- could they be given a positive charge and create salts in particular perchlorates?
Would that work with tetranitro phenazine as well?
Are you sure it would really be that wasteful? mad science is about exploring new substances... even IF a certain reagent is slightly impractical. Its
not like were building a truck-bomb; we want to explore new energetic (and other) horizons!
  
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well I think that's an interesting reaction.
Also, with the final product, shouldn't it be possible to form salts, i.e. nitrate or perchlorate, similar to pyridine perchlorate?
Also, reducing the bonds on the nitrogen, and subsequently forming the nitramine should make this very interesting....
But let's not turn this into a theorising thread... 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
Would it be possible to react p-dinitrobenzene with p-diaminobenzene to form long chains? Or at least p-dinitrobenzene with aniline?
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by cumbustion
Are you sure it would really be that wasteful? mad science is about exploring new substances... even IF a certain reagent is slightly impractical. Its
not like were building a truck-bomb; we want to explore new energetic (and other) horizons!
|
Im all for "explore new energetic (and other) horizons!" thats what this place is about, but no reasion to make it as impractical as
possible.
3,5-dinitroaniline is the hardest to get to, nitration of aniline will result in 2,4-dinitroaniline & getting to trinitrobenzene is no easy feat.
So, the information you seek regarding nitration of phenazine should be in the article I linked to, but i dont have it. Starting with phenazine is
sooo much easier then TNB/3,5-DNA which may not work at all, and the reactions involved are already documented, no guessing involved. The original
article for the wohl aue reaction is on the page you first linked to "A. Wohl and W. Aue, Ber. 34, 2442 (1901)".
| Quote: | Originally posted by cumbustion
Would it than be a waste to start a thread on tetranitrophenazine or similar substances? |
No need to start yet another thread, just appeal to a moderator to change the title of this one to "phenazine & derivatives" or words to
that effect.
[Edited on 28-8-2005 by Axt]
|
|
|