greenyppols
Hazard to Self
 
Posts: 97
Registered: 17-3-2005
Location: Behind you, watching intently, sexually tense.
Member Is Offline
Mood: Who's been messin' with my moods?
|
|
Thermometers
I dont see anything about thermometers on the board here, so I was wondering if anyone has preferences?
N.I.S.T for accuracy, I can obviously think of.
Anyone use digital thermometers? And how, if you do use it, do you place the probe? Same place you would the bulb of a thermometer? Or is this why
I've never seen a digital probe sticking in a flask? so far anyway. Im wondering because a ground glass thermometer I waited for WEEKS for
finally came, and in my joy of finally receiving this precious new 'dead balls-on accurate' addition (I just watched 'My Cousin
Vinny' ), and in sitting in a cushioned couch with it in my hand, holding
it in the center of it's plastic-tube encased self, proceeded to snap it in half and bust it. ), and in sitting in a cushioned couch with it in my hand, holding
it in the center of it's plastic-tube encased self, proceeded to snap it in half and bust it. 
Ohhhhh, the $$$ that just flew out the window. Im honest, Im not going to blame my stupidity and claim it was shipping damage. Karma, ya know? lol.
So that explains my reason for this questioning post and leads me to another question I'll ask without starting another thread.
I know this was a mercury thermometer. It had red warning lables all over the tube. I did not even open it. I left it closed and even put some
electrical tape on the cap to make sure it stayed sealed, put it in 3 ziplock baggies in a tupperware container, where it now sits outside with a
cinder block on it. What/where is the best way to safely dispose of this now? Breaks my heart I never laid eyes on it.
Im not gonna pollute, because I give a hoot. 
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Your environmentalism inspires me.
lol, no. It doesn't... I believe I'm with a large percentage of the board when I say that mercury has done <html><i>me</i>
no harm! If you were to swallow it, it would probably do you little harm... the MSDS these days are apophrycal, and worth little attention.
As Robert De Nero of Ronin fame says, "a [thermometer] is a tool, it's job is to get things done" for that reason I say that all
thermometers are equal.
I have a long history of distilling. I used digital thermometers for a while, attracted by their sexy slimline appeal and precise measurements. I soon
found, however, that alcohol thermometers are just as good or superior to digital ones - they do the same job! Digital thermometers are meant for
simpletons - they are just the same as regular thermometers, except more expensive and less accurate (the only windfall being user interface [much
like windows, come to think of it!] )
So in summary - I have found that digital and 'analogue' thermometers are really no ddifferent. The type of thermometer you use is based
solely upon the range of temperatures you want to measure. Sux 2 u 4 br8kng ur th3rmmtr. I feel your pain.</html>
Caveat Orator
|
|
|
greenyppols
Hazard to Self
 
Posts: 97
Registered: 17-3-2005
Location: Behind you, watching intently, sexually tense.
Member Is Offline
Mood: Who's been messin' with my moods?
|
|
Yeah yeah, I know. Overboard. As a kid I used to swipe some mercury that my grandfather kept in like a..half gallon glass bottle...at least it seemed
like a half gallon size to me.
Just watched it roll around the shed floor. He'd get some po'd if he ever caught me and I'll leave out the bloody details. I also
compared the freezing points of india ink and mercury on a slab of dry ice once..some school experiment that my moms friend showed me....
...and despite all that, Im alive. I'll argue any peculiarities I may have exist only as a figment of your imagination....
But..it still ain't gettin' out. At least on my property at least..NIMBY self serving.
I read sulphur soaks it up and makes it harmless? I can pop the cap off and dump some in, I guess. or do I have to get into it more than that. Im
sure its mostly still in the bulb. It sounded like it busted towards the end away from the bulb.
[Edited on 29-9-2005 by greenyppols]
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by greenyppols
I read sulphur soaks it up and makes it harmless? I can pop the cap off and dump some in, I guess.
[Edited on 29-9-2005 by greenyppols] |
In the mercury spill kits I've seen in my chemistry labs at the university I go to, they mostly have some sulfur in there.
AFAIK, the sulfur absorbs the mercury to form mercuric sulfide, which is pretty much insoluble. Then from there, you can collect the solid waste and
store it indefinitely, or properly dispose of it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I had 2 mercury thermometers broken in shipment from a well-known science equipment supplier. I called my local hazardous waste person ( a city
official) and asked him what to do with them. He said just take them out to the hazardous waste station (at the city dump) and place them on the
table. They get them all the time. End of problem.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
I thought it was granular zinc in those mercury spill kits. It forms a solid alloy that can be swept up. There is also so sort of sponge that is
used. If it is in the air, crank the heat up to 80 and open all the windows, and leave for a day. It may sound like a waste of energy, but the
mercury left will vaporise and leave the house via the windows. It doesn't sound like you got enough spilt to worry.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
A word on NIST thermometers
Not all thermometers are created equal. For example, take a look at this one. I looked at it and thought that it was one of those thermometers that met the NIST standard of always being within 0.1*C of the actual
temperature. As it turns out, the thermometer only ‘conforms to standards traceable to NIST’, i.e. it’s within 1% of temperature range away from
the true temperature, i.e. +/- 1.7*C. In actuality, those cheap Ertco thermometers Cynmar sells are always closer to the true temperature than that
‘NIST’ thermometer I got. The Ertcos say water boils at 100-102*C, the ‘NIST’ says 106*C.
Make sure you know what you’re really buying. Just because it says NIST doesn't mean it's a particularly good thermometer.
|
|
|
greenyppols
Hazard to Self
 
Posts: 97
Registered: 17-3-2005
Location: Behind you, watching intently, sexually tense.
Member Is Offline
Mood: Who's been messin' with my moods?
|
|
Neutrino, thats what I was wonderin'.
So whats a good accurate brand? I have 3 alcohol therms that read boiling water at 98c and the other at 104c and other that reads 100c as it should.
But how the hell would I know?? Thats fairly good for a lot things below 100c I figure, I can make adjustments, but if it gets out of whack the higher
it gets, I'd have no clue what was what.
Besides, I'd rather it just be accurate, and KNOW it, and be done with it.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
You could always calibrate your thermometer by testing it against solids with known melting points. Of course, not many of us have our own melting
point apparatus, but if you live near a university there is usually a 'melting point room' open to students, but the door is always open if
you know what I mean.
|
|
|
Slanesh8116
Harmless

Posts: 1
Registered: 3-10-2005
Member Is Offline
Mood: No Mood
|
|
I see the benefits of getting a analogue thermometer with it being glass and non reactive as appose to being metal and reacting with pretty much
everything, however I searched for an analogue thermometer but apart from 'liberating' I wasn’t going to be able to get hold of one easily
so I just got a digital one that claims to be 0.1*c accuracy so I am not complaining because I simply don’t require anything more accurate than 1-4
degrees out, so digitals are newbie tools while glass analogue are more for the coinsures of chemistry
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I needed a couple of thermometers with small, flexible tips to be inserted in orifices of my Ranque-Hilsch tests (to be posted soon). This is as quick
and cheap as I could get:
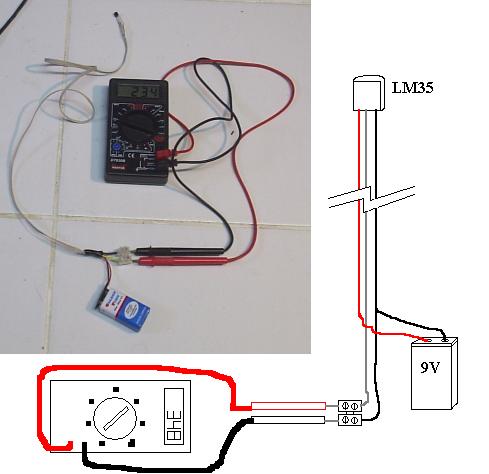
The LM 35 looks like a transistor and can be powered from 4 to 40 V. I used a 9v battery. It's output is, in milivolts, it's temperature X 10 in °C.
The range is... well, google for the datasheet.
A cheap digital multimeter becomes a digital thermometer.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Tacho: Love your design for its simplicity. IIRC you should connect one resistor between battery and LM35 as work current of this chip should not
exceed 5mA. 3k resistor for 9V battery should do.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
chromium,
None of the typical applications in the datasheet seem to use a resistor, but since it's current drain is rated "less than 60uA" (yeah, microamperes),
a resistor should do no harm. Both my units are connected directly to 9v batteries and seem to be doing fine.
I didn't do the math neither the tests, but with 60uA current drain, a standart 9 volts battery should last a bit less than eternity.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Seems that you are right. I looked LM135 data sheet for short reference. LM135 needs current limiting resistor. It appears to be similar to LM35 but
not exact analogue as i thought it is.
[Edited on 4-12-2005 by chromium]
|
|
|